Key Points
- •
Seronegative spondyloarthropathies are interrelated chronic inflammatory rheumatic diseases that include ankylosing spondylitis (AS), reactive arthritis, psoriatic spondyloarthropathy, spondyloarthropathy associated with inflammatory bowel disease (IBD), and undifferentiated spondyloarthropathy.
- •
Seronegative spondyloarthropathies are characterized by sacroiliitis with or without spondylitis, peripheral oligoarthritis, enthesitis, dactylitis, and inflammation of nonarticular structures.
- •
These disorders occur in genetically predisposed individuals and are triggered by environmental factors.
- •
HLA-B27 is present in up to 95% of patients of European ancestry with AS and is seen in the majority of patients with reactive arthritis, spondylitis associated with IBD, and psoriatic spondylitis.
- •
Bilateral, symmetric sacroiliac involvement is characteristic of AS but may be seen in any of these disorders and occasionally in other conditions.
- •
Unilateral or asymmetric sacroiliitis suggests psoriatic arthritis or reactive arthritis. Infection should be excluded in cases of unilateral sacroiliitis.
- •
Magnetic resonance imaging is preferred over conventional radiography and computed tomography because of its ability to detect early inflammatory changes.
- •
Spondylitis begins at the discovertebral junctions, resulting in erosions, squaring, “shining corners,” and, eventually, bony bridging (syndesmophytes).
- •
Complications of AS include acute spine fracture, chronic discovertebral lesions, and cauda equina syndrome.
- •
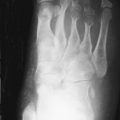
Psoriatic arthritis typically involves the distal interphalangeal joints with erosion or ankylosis. New bone formation occurs adjacent to the erosions. Osteoporosis is atypical. Periosteal reaction may occur along the phalanges and bony prominences. A “sausage digit” is a characteristic feature.
- •
The radiographic features of enteropathic spondyloarthropathy are identical to those of ankylosing spondylitis.
The seronegative spondyloarthropathies are a group of heterogeneous, interrelated chronic inflammatory rheumatic diseases that include ankylosing spondylitis (AS), reactive arthritis, psoriatic spondyloarthropathy, spondyloarthropathy associated with inflammatory bowel disease (enteropathic) (IBD), and undifferentiated spondyloarthropathy.
Seronegative spondyloarthropathies include AS, IBD, reactive arthritis, psoriatic spondylitis, and undifferentiated spondyloarthropathy.
These diseases are characterized by sacroiliitis with or without spondylitis, peripheral joint oligoarthritis, enthesitis, dactylitis, and inflammation of nonarticular structures (e.g., skin, gastrointestinal [GI] tract, eye, and heart).
Although knowledge of the cellular and molecular mechanisms of inflammation in these diseases is incomplete, the data suggest they are multifactorial processes occurring in genetically predisposed individuals, which are triggered by environmental factors. Thus our current understanding of the spondyloarthropathies converges along three lines of investigation: (1) clinical and epidemiologic studies, in which diagnostic imaging has played an important role; (2) genetic and immunologic studies, focusing on an inherited predilection to developing spondyloarthropathy; and (3) studies of infectious agents that may act as the environmental triggers for the development of spondyloarthropathy.
An unresolved entity that also appears linked to the seronegative spondyloarthropathies is SAPHO syndrome ( s ynovitis, a cne, p ustulosis, h yperostosis, and o steitis), in which a significant number of patients fulfill accepted criteria for spondyloarthropathy but do not usually have the genetic predispositions found in patients with the other seronegative spondyloarthropathies.
SAPHO: s ynovitis, a cne, p ustulosis, h yperostosis, and o steitis.
CLINICAL DATA AND EPIDEMIOLOGY
The anatomic and clinical reports at the end of the nineteenth century by von Bechterew, Strumpell, and Marie are generally credited as the first significant descriptions of AS, and these reports served to establish AS as a distinct disease entity.
Over the course of the twentieth century, our understanding of AS has been further augmented by the evolution of radiographic techniques. In 1937, Andersson described discovertebral destructive changes representing a spondylodiscitis identified on radiographic examinations of two patients with AS. In 1952, Romanus reported on the radiographic appearance of vertebral margin erosions of bone followed by sclerosis and ossification occurring in patients with AS. In 1956, Forestier et al. described additional radiographic manifestations of AS, including sacroiliitis and syndesmophyte formation. These reports showed the importance of the imaging features of AS, which today remain integral to the diagnosis and staging of the spondyloarthropathies. In the latter half of the twentieth century, the development of more sophisticated imaging techniques such as computed tomography (CT) and magnetic resonance imaging (MRI) has led to numerous reports of improved image sensitivity and specificity for the diagnosis of spondyloarthropathies.
In North America during the midportion of the twentieth century, the concepts of “rheumatoid spondylitis” and “rheumatoid variant” were used to indicate that AS was thought to be a variant of rheumatoid arthritis (RA) affecting the spine. During this same period, there were also clinical, radiographic, and epidemiologic reports showing relationships between AS and other types of arthritis. In 1960, Graham was among the first to suggest that AS was a separate entity from RA. In 1974, Moll et al. suggested that the “rheumatoid variants” were discrete entities and introduced the concept of the “seronegative spondyloarthropathies” on the basis of overlapping clinical and radiologic features within a number of different diseases that shared clinical features distinct from RA. Specific characteristics that were noted to distinguish the spondyloarthropathies from RA were seronegativity for rheumatoid factor, absence of nodules, and radiologic evidence of sacroiliitis. The original grouping of spondyloarthropathies included AS, Reiter’s syndrome (i.e., reactive arthritis), psoriatic arthritis, juvenile spondyloarthropathy, and arthritis associated with inflammatory bowel disease (IBD); Whipple’s disease and Behçet’s syndrome were also incorporated into this grouping but were subsequently excluded.
In 1990, Amor et al. proposed a classification of the spondyloarthropathies using various criteria with a scoring system to establish a diagnosis of spondyloarthritis. In 1991 the European Spondyloarthropathy Study Group (ESSG) broadened these criteria for diagnosis to accommodate undifferentiated forms of spondyloarthropathy (i.e., patients who manifest features of, but fail to fulfill criteria for, the other spondyloarthropathies) and included findings such as seronegative oligoarthritis, dactylitis or polyarthritis of the lower extremities, and heel pain due to enthesitis, which had been ignored in previous epidemiologic studies. In addition, patients with acute anterior uveitis and HLA-B27 positive individuals with spondylitic heart disease (i.e., complete heart block and/or aortic regurgitation) were considered to be within the spectrum of the seronegative spondyloarthropathies. Using these criteria, one epidemiologic study reported undifferentiated spondyloarthropathy to be second in frequency only to AS.
GENETICS AND IMMUNOLOGY
Identification of the human leukocyte antigen (HLA) in the 1950s was the initial important step leading to characterization of the human major histocompatibility complex (MHC), later shown to be located on chromosome 6 and responsible for encoding the histocompatibility antigens that are divided into Class I, II, and III genes. This work would subsequently provide a foundation for understanding the genetic predispositions of individuals who develop spondyloarthropathies. A clear correlation has now been documented between patients with spondyloarthropathy and the presence of HLA-B27, which is present in up to 95% of patients of European ancestry with AS, 70% with reactive arthritis, 70% with spondylitis associated with IBD, 60% with psoriatic spondylitis, and 50% with acute anterior uveitis occurring without other features of spondylarthropathy. Studies have also shown a remarkable familial aggregation of these disorders; 20% of HLA-B27 positive relatives of patients with ankylosing spondylitis are ultimately affected and a concordance rate as high as 63% has been shown in identical twins versus 23% in nonidentical twins. These statistics, however, provide only limited insight into the complex genetic factors influencing the development of the spondyloarthropathies: Fewer than 5% of individuals who are HLA-B27 positive will develop a spondyloarthropathy, and multiple other MHC genes (e.g., HLA-DRB1) and molecular subtypes of HLA-B27 have now been implicated in the development of AS, including HLA-B*2705, HLA-B*2704, and HLA-B*2707.
Fewer than 5% of HLA-B27-positive individuals develop spondyloarthropathy.
In contrast to patients with AS who have a high frequency of HLA-B27, patients with psoriatic arthritis have a higher frequency of HLA-B17, HLA-B39, and HLA-Cw6.
ASSOCIATED INFECTIOUS AGENTS
In 1916, Reiter described a syndrome of non-gonococcal urethritis, peripheral arthritis, and conjunctivitis following dysentery. This account presaged subsequent interest in the role of infection in the etiology of the spondyloarthropathies. Subsequent reports by Bauer et al. and Paronen documented a more specific relationship between the development of Reiter’s syndrome and prior genitourinary or GI infections. In 1969, Ahvonen et al. reported on patients with arthritis following GI infection with Yersinia enterocolitica and proposed the concept of reactive arthritis. Other bacteria implicated in the development of reactive arthritis include genitourinary infections with Chlamydia trachomatis and enteric infections with gram-negative organisms such as Shigella, Salmonella , and Campylobacter . Additional evidence of this association is the finding of antigens for Salmonella, Yersinia , and Chlamydia in synovial tissue and fluid from patients with reactive arthritis. In 1978, Ebringer et al. implicated enteric Klebsiella pneumoniae infection as a trigger in the pathogenesis of AS, although this has never been confirmed, and a more recent report by Stebbings et al. implicated Bacteroides as a trigger for AS.
There has also been considerable speculation about the etiologic role of infection to explain the association between AS and IBD, presumably resulting from breakdown of the mucosal barrier of the gut allowing intestinal bacteria to stimulate the immune system. This association is more common than past reports would indicate and, in particular, it has been shown that over 50% of patients with spondyloarthropathy have evidence of silent IBD documented by endoscopic examination. Furthermore, de Vlam et al. reported that 30% of 103 patients with either ulcerative colitis or Crohn’s disease had symptoms of inflammatory back pain, 90% fulfilled diagnostic criteria for spondyloarthropathy, and 18% had asymptomatic sacroiliitis.
Multiple reports have drawn attention to important links among immunity, infectious agents, and psoriasis. Prinz has shown that, in some patients, psoriasis vulgaris is the result of a sterile antibacterial skin reaction initiated by streptococcal T cells that cross-react against epidermal autoantigens. Not surprisingly, human immunodeficiency virus (HIV) has also been reported in association with psoriatic arthritis, reactive arthritis, and patients with undifferentiated spondyloarthropathy, and McGonagle et al. have shown that patients with HIV have a tenfold increased frequency for developing psoriatic arthritis.
SAPHO SYNDROME
In 1987, Chamot et al. suggested the acronym SAPHO to describe a group of 85 patients with pustular acne and hyperostotic inflammation of bone. The characteristic feature of this syndrome is osteitis, usually with negative bacterial cultures, with or without skin lesions. Reports of other entities with an association between cutaneous disease and musculoskeletal inflammation that are likely part of the spectrum of SAPHO syndrome include: sternocostoclavicular hyperostosis, pustulotic arthroosteitis, chronic recurrent multifocal osteomyelitis with pustulosis palmaris et plantaris, acne conglobata with spondyloarthropathy, and hidradenitis suppurativa. Of related interest is a study of clinical subsets in patients with psoriatic arthritis in which 2% of patients showed features of SAPHO syndrome. Maugars et al. have suggested that psoriasis is the “link” between SAPHO and the spondyloarthropathies.
SAPHO more commonly affects women than men and the clinical course is variable, with some patients experiencing continuous bone and joint symptoms, whereas others may show intermittent episodes of disease. Although individuals with SAPHO syndrome have only a slightly increased incidence of HLA-B27, a significant number of patients exhibit diagnostic criteria for seronegative spondyloarthropathy, with a reported frequency of spondylodiscitis between 9% and 32%. The spine is second only to the sternocostoclavicular area as the most common site of involvement in adults, and spondylodiscitis may, in fact, be the initial presentation of SAPHO syndrome.
Like other spondyloarthropathies, an association between infection and the development of SAPHO syndrome has been proposed. Although bacterial cultures are usually negative, there have been multiple reports documenting P. acnes as a cause of discitis, osteitis, and arthritis, supporting a potential link between this organism and SAPHO syndrome.
OSTEOARTICULAR FEATURES AND IMAGING
Ankylosing Spondylitis
AS is the most common inflammatory spondyloarthropathy and represents the prototype for all the other spondyloarthropathies. The diagnosis of AS requires clinical suspicion and astute patient assessment, with medical imaging subsequently playing an important role in confirming the diagnosis.
Patients with AS usually present with back pain and stiffness in adolescence or early adult life, typically commencing in the second or third decade of life, but onset after age 45 may occur. In fact, diagnosis after the age of 45 is not uncommon because symptoms are often insidious and the diagnosis may be overlooked. To further complicate this issue, it has been suggested that late-onset undifferentiated spondyloarthropathy occurs more frequently than AS after the age of 50 and that late-onset spondyloarthropathy is not rare.
Men are two to three times more commonly affected than women, although the prevalence in women has likely been underestimated in many reports because women tend to have less severe involvement of the spine and more symptoms affecting the knees, wrists, ankles, hips, and pelvis. The clinical picture with juvenile onset of disease is also different from that of adult onset by the more frequent occurrence of peripheral joint disease.
A long-term follow-up study by Carette et al. showed that the natural history of AS is generally established in the first 10 years of disease and that the presence of peripheral joint involvement, especially of the hip, portends a poorer prognosis.
The imaging hallmark of AS is sacroiliitis, and radiographic studies remain the first line of investigation, looking for both the destructive and proliferative changes of bone associated with structural joint damage.
Sacroiliitis is recognized as the earliest feature of AS. During the initial course of the illness, sacroiliac disease may be asymmetrical, or occasionally unilateral, but with time, the distribution customarily evolves to produce bilateral, symmetric involvement.
Bilateral, symmetric sacroiliitis is characteristic of AS but not specific.
Both the synovial and ligamentous portions of the sacroiliac (SI) joints are affected, although in the earlier stages of the disease, the iliac side of the joint classically shows greater alteration than the sacral side. The initial manifestations include periarticular osteopenia with ill-definition of the joint margins accompanied by subchondral sclerosis, primarily on the iliac side of the joint; the joint space may also appear variably widened, although discrete erosions may be difficult to identify.
Erosions involve the iliac sides of the SI joint first.
As the process becomes more advanced, subchondral sclerosis becomes evident on both sides of the joint, erosions become more distinct, and narrowing of the joint space may occur with areas of incomplete bony bridging. The end stage of this process is complete ankylosis of the SI joints, often with loss of the bony eburnation observed in earlier stages of the disease. Other features that may be seen in the pelvis and hips that frequently accompany these findings are ossification of the SI and lumbosacral ligaments, abnormalities of the pubic symphysis, hip arthritis and inflammatory enthesopathy of the iliac crests, ischial tuberosities and femoral trochanters ( Table 23-1 ).
Based on these features, the New York criteria were developed for grading sacroiliitis and are as follows: 0 = normal; 1 = suspicious (no definite abnormality); 2 = minimal (loss of definition of the SI margins, some sclerosis, minimal erosions) ( Figure 23-1 ); 3 = moderate (sclerosis on both sides of the SI joints, indistinct margins, erosions, joint space loss) ( Figure 23-2 ); and 4 = severe (ankylosis) ( Figure 23-3 ).




With the advent of effective treatment for AS, the need to measure structural damage and its progression has become essential. As a result, other radiographic scoring methods, such as the Bath Ankylosing Spondylitis Radiology Index, the Stoke Ankylosing Spondylitis Spine Score (SASSS), and the modified SASSS (M-SASSS), have been developed and tested to evaluate radiographic evidence of disease progression. One recent study concluded that the M-SASSS is the most appropriate for scoring disease progression, but a 2-year radiographic study of these scoring methods in patients with AS concluded that, although these methods are moderately to excellently reliable, change was too small to be reliably detected in the 2-year time frame.
Although scintigraphy may occasionally be helpful for evaluation of unilateral sacroiliitis, its role is limited because it has low sensitivity and specificity and generally does not alter decisions about disease probability.
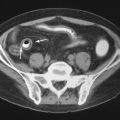
For patients in whom clinical suspicion of early disease is high, yet radiographic studies are negative or equivocal, computed tomography (CT) and magnetic resonance imaging (MRI) are important in documenting the presence or absence of sacroiliitis. Prior to the introduction of MRI, Kozin et al. showed that CT is more sensitive than radiography and is equally specific for identifying sacroiliitis ( Figure 23-4 ). In HLA-B27 patients with clinical evidence of sacroiliitis, 50% of these individuals had negative or equivocal radiographs whereas only 19% had negative CT images for sacroiliitis. Fam et al. also found that CT improved delineation of the SI joints in early AS and revealed more abnormalities and higher grades of sacroiliitis than conventional radiography ( Figure 23-5 ). Similarly, CT showed the presence of asymptomatic sacroiliitis in 32% of 65 patients with known IBD, compared with conventional radiography, which was positive for sacroiliitis in only 18% of patients. More recent investigation has shown that CT is equally as accurate as MRI in defining erosions of the SI joints but is ineffective in detecting synovial inflammation; thus MRI permits differentiation between active and chronic sacroiliitis.





MRI has proven even more valuable than CT in the early detection of inflammation and destructive changes involving the spine and SI joints, and it is generally preferred over conventional radiography and CT because of its ability to detect the early inflammatory changes of bone and synovium ( Figure 23-6 ). An understanding of the normal MRI appearance of the SI joints is mandatory for accurate interpretation of abnormalities affecting these joints, and axial images provide superior delineation of the normal anatomy and abnormalities of the SI joints. MRI findings in early disease show that sacroiliitis is most often bilateral, affects the iliac side of the joint more frequently than the sacral side, and the inflammation is most frequent in the dorsocaudal parts of the synovial joint and bone marrow. A prospective study comparing radiography, quantitative SI scintigraphy, and MRI for the detection of sacroiliitis in 44 patients with inflammatory low back pain found MRI to be the most sensitive imaging technique (i.e., 95% sensitivity compared with 48% for scintigraphy and 19% for conventional radiography). Thus MRI combined with various scoring techniques has now become an integral part of more recent studies and clinical trials that seek to quantify disease activity and measure response to treatment ( Figure 23-7 ).




The most commonly employed MR pulse sequences for identifying inflammatory disease in the spine are short tau inversion recovery (STIR) images in the coronal plane and gadolinium-enhanced, T1-weighted, fat-saturated images in the axial and coronal planes; unenhanced T1-weighted and fat-suppressed T2-weighted images have also been included in several studies. One investigation showed that more spinal lesions were detected with the STIR sequence but that reader reliability was better with the gadolinium-enhanced sequences.
MRI methods for grading SI disease reflect those previously developed with the New York criteria and include assessment of erosions, bony sclerosis, and joint space width; however, additional features that can be evaluated include bone marrow edema and gadolinium contrast enhancement. In a recent study by Puhakka et al., scores for disease severity were obtained by grading each finding as: 0 = normal, 1 = minimal, 2 = moderate, or 3 = severe. An overall score for joint destruction was obtained as the sum of the scores for erosions, sclerosis, and joint width. Similarly, an overall score for inflammation was obtained as the sum of scores for bone marrow edema and gadolinium contrast enhancement of the marrow and joint space. These two overall scores were then considered reflections of chronicity and acuity of disease, respectively. Another important conclusion made by this report was the greater sensitivity of MRI in detecting disease progression during the 1-year period of the study compared with the sensitivity of radiographs, which require about 4 years of follow-up to detect progression.
In the spine, abnormalities first appear at the thoracolumbar and lumbosacral junctions and later progress along the remainder of the spine ( Figure 23-8 ). Osteitis is the earliest expression of spinal involvement, manifesting as focal areas of bone erosion at the discovertebral margins. When accompanied by productive new bone filling the concavity of the anterior vertebral surface, the vertebrae assume a squared appearance, most notably in the lumbar spine ( Figure 23-9 ). Sclerosis develops with healing of the erosions, producing the so-called “shiny corner” sign.


Early findings of spondylitis are “shining” corners and vertebral squaring.
This bone formation then extends along the discovertebral margin, forming vertically oriented ossification of the annulus fibrosus, referred to as syndesmophytes . This pattern of ossification can further extend along the anterior longitudinal ligament, as well as the paravertebral soft tissues ( Figure 23-10 ).

Patients with AS may develop cauda equina syndrome, and previous myelographic studies have shown the presence of arachnoid diverticula in these patients associated with a capacious dural sac. Although myelography is rarely performed in modern clinical practice, it should be noted that laminar erosions related to these diverticula may be identified with CT and MRI.
Cauda equina syndrome (low back pain, sciatica, leg weakness, sensory disturbance, loss of bladder and bowel function, and saddle anesthesia) may occur in AS.
A report by Braun et al. showed that the thoracic spine is a more common site of spinal disease than either the lumbar or cervical spine. They emphasized that recognition of this factor is important because most radiographic scoring systems do not include this spinal segment, and they added that intrarater and interrater variation of radiographic interpretations was greatest in the thoracic spine. As a result, Braun et al. 83 concluded that thethoracic spine is best assessed by MRI, which has an essential role in defining chronic changes in the thoracic spine.
The most serious complication of AS is spine fracture, which can occur, even with minor trauma, due to the rigidity and osteopenia that develop in the spinal column ( Figure 23-11 ). The most frequent sites for fracture are the cervical spine, with the risk of quadriplegia, and the thoracolumbar junction. Fractures that develop in AS patients are associated with high morbidity and mortality rates.

Patients with AS may also develop sclerosis and destructive changes of the discovertebral junction, usually unrelated to infection, and these changes have been termed Andersson lesions , named after the investigator who originally described them ( Figure 23-12 ).

Development of new pain in a patient with late-stage AS suggests “pseudarthrosis.” Demonstration of a defect in the posterior elements, as well as anteriorly, helps confirm abnormal motion.
Although the etiology of this abnormality remains controversial, various explanations have been proposed, including inflammation, a persistent motion segment (pseudarthrosis), fracture, and infection.
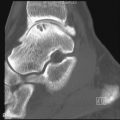
The hip, shoulder, and knee are the most important sites of peripheral arthritis in AS. In the hip, one of the earliest features of arthritis is the development of osteophyte formation at the margin of the femoral head often associated with joint space loss and variable subchondral cystic changes ( Figure 23-13 ).