Introduction
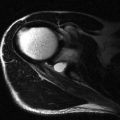
Shoulder pain is one of the commonest orthopaedic presentations in the general population and subacromial impingement is a common underlying cause. The glenohumeral joint is an intrinsically unstable joint and the tendons of the rotator cuff play an important role in stabilizing it ( Fig. 2.1 ). The supraspinatus tendon (SST), the most important component of the rotator cuff, lies along the superior aspect of the humeral head passing beneath the coracoacromial arch. The arch is made up of a bony component posteriorly, the acromion, and a soft tissue component anteriorly, the coracoacromial ligament. The other tendons of the rotator cuff are subscapularis, anteriorly, and infraspinatus and teres minor, posteriorly.
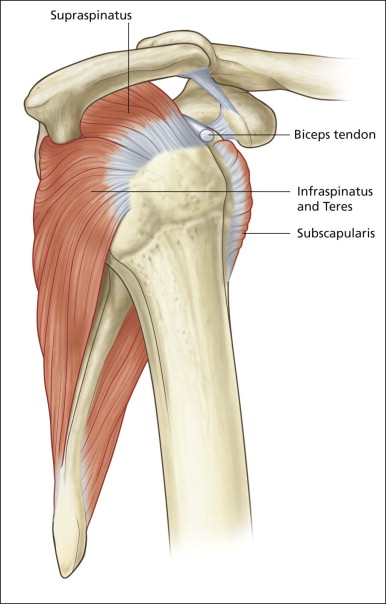
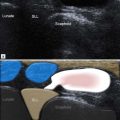
The other important anatomical structure involved in shoulder impingement is the subacromial subdeltoid (SASD) bursa. This is a large bursa that also passes underneath the coracoacromial arch separating it from the rotator cuff below. It also separates the cuff from the deltoid muscle laterally and a component extends medial to the arch between the supraspinatus muscle belly and trapezius above. Although large, the bursa is quite thin, containing only a small quantity of fluid under normal circumstances. Its function is to facilitate movement of the SST beneath the acromion and the coracoacromial ligament.
Patients with external impingement syndrome, painful arc or, as it is more usually referred to: impingement syndrome, typically present with insidious onset shoulder pain and limitation of movement. Pain occurs in a variety of classical arm positions, including working with the arm above the head, trying to put on a shirt or undo a bra strap or reaching to the backseat of a car. In the early stages, these movements induce pain that settles, but in time becomes more persistent with repeated insult. The patient begins to wake at night when they turn onto the affected shoulder, sleep is disturbed and ultimately pain becomes continuous. Classically, the patients point to the lateral aspect of the deltoid as the location of symptoms. Weakness may be present and related to muscle disuse or tendon tears.
The principal pathology is SASD bursitis with or without supraspinatus tendinopathy or tear.
The bursal wall becomes thickened and pain and limitation of movement occur as the inflamed bursa and SST pass under the coracoacromial arch on shoulder abduction.
External impingement must be distinguished from internal impingement syndromes, which generally refer to disorders of the glenoid labrum such as superior labral tear from anterior to posterior (SLAP) tears and posterosuperior impingement. Pain due to sternoclavicular arthropathy is sometimes referred to as tertiary impingement. Ultrasound has a minimal role in the former but is useful in detecting and treating the latter.
The Aetiology of Shoulder Impingement
As with tendinopathy elsewhere in the body, a combination of extrinsic and intrinsic factors, coupled with misuse and combined with a genetic predisposition, lead to tendinopathy and rotator cuff tears. Extrinsic factors include external impingement on the tendon from the surrounding bony or soft tissue components of the arch.
Bony Impingement
Multiple factors have been implicated in the aetiology of impingement and rotator cuff disease. Attention is frequently drawn to the shape of the acromion, although it is now generally accepted that, apart from extremes, changes in the acromion are secondary to impingement rather than a primary cause. Bony irregularity on the undersurface of the acromion can arise as a result of enthesopathy at the coracoacromial ligament attachment. In extreme cases, a distinct bony spur may be present, leading to further narrowing of the subacromial space. Entheseal changes may also occur at the humeral insertion of the involved tendons. Bony upgrowth from the humeral head combines with bony downgrowth from the acromion leading to further diminution in the subacromial space, increased bursal impingement and progressive supraspinatus tendinopathy ( Fig. 2.2 ). Although bony and entheseal factors have been shown to narrow the subacromial space, it is probable that they are not the most important.

SICK Scapula
Two factors that are almost certainly more important than acromial shape and enthesopathy in the aetiology of rotator cuff disease are genetic factors and scapulothoracic dyskinesia.
Other Theories
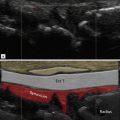
An important intrinsic mechanism leading to tendon degeneration and tear is apoptosis: the intrinsic degeneration of collagen, largely influenced by genetic factors. Like the stress factors of bone, the state of a tendon at any one time is a balance between damage and repair. If apoptosis is accelerated beyond the intrinsic repair mechanism’s ability to heal, tears occur. A watershed area has been defined in an area of the tendon close to, but not at the bony attachment, where an area of decreased vascularity may predispose to cuff tear. This area is often referred to as the ‘critical zone’. Anatomical studies, however, have not always demonstrated an area of decreased vascularity, nor is it obvious that the majority of tendon tears occur in this area. Decreased vascularity has not been consistently demonstrated and, when it is seen, it is not clear that this is not an effect rather than the cause of tendinopathy. In many diseased tendons in the body, prominent increased vascularity is present. Notable examples include the Achilles and patellar tendons. Neovascularity or angioneogenesis is not a prominent feature in supraspinatus tendinopathy. The underlying cause for the differences between these tendons is unclear.
The Rotator Cable
It has been suggested that central or ‘crescent’ supraspinatus tears are more common in older individuals as a degenerate phenomenon. It has also been suggested that they are more likely to occur when there is a prominent condensation of fibres traversing the supraspinatus, called the rotator cable. The rotator cable has not been extensively studied, but it appears to be a condensation of tendon fibres that are orientated in a different plane from the remainder of the tendon. Together they form a band of tissue that extends as an arch from the anterior leading edge into the tendon substance to the posterior margin of the tendon close to its insertion. The cable reinforces the anterior and posterior parts of the tendon in the same manner as a cable links the struts of a suspension bridge. Between the cables, a weak area is created in the central substance of the tendon, secondary to stress shielding. This area becomes more prone to degeneration leading to a tear. The terms central, midsubstance or cresent have all been applied to this tear pattern. It has also been suggested that the presence of a strong rotator cable may explain why not all tears are symptomatic.
Supraspinatus Tear Nomenclature
Partial Versus Full Thickness
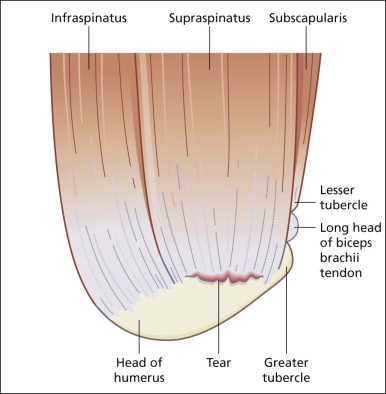
One of the issues that frequently causes confusion is the nomenclature of cuff tears, particularly the definition of partial versus full thickness versus massive rotator cuff tears. This can be more easily understood when it is appreciated that the SST is a sheet-like tendon, unlike, say, the tendons of the wrist that are more tubular or string-like. As such, the supraspinatus has both thickness and width . It has a superior surface facing the subacromial bursa and an inferior surface facing the glenohumeral joint. An intact SST prevents communication between these two compartments. Its anterior edge abuts the biceps/rotator interval and its posterior margin blends with the infraspinatus tendon into which tears may extend.
If a tear is detected, its description should include a comment on its thickness, width and location.
A partial thickness tear is one that involves either the joint or bursal surface alone and does not allow communication between the two compartments ( Figs 2.3 to 2.5 ). If contrast is injected into the glenohumeral joint, it will fill a joint surface partial tear but will not pass into the SASD bursa. If contrast is injected into the bursa, it will fill a bursal surface partial tear but will not pass into the joint. The most common tear pattern is said to be a joint surface partial tear, although some authors say that this is due to underdiagnosis of bursal surface lesions. A full-thickness tear is present when both surfaces are involved and the tear results in communication between the joint and the bursal compartments. Once this communication is present, a full-thickness tear is diagnosed regardless of the width of the communicating channel. Some full-thickness tears are of large width, measuring greater than 3 cm in diameter, others are little more than pinholes. A full-thickness tear might also be described as extending from the anterior leading edge with 1 cm of supraspinatus remaining intact or as involving the midportion with 1 cm of supraspinatus intact anteriorly and 1 cm of infraspinatus intact posteriorly, and so forth.



Full-Thickness Tear Patterns, Location and Shape
Supraspinatus is divided into an anterior portion, sometimes called the leading or free edge, and a posterior portion, also called the crescent, midsubstance or footprint. Occasionally a posterior portion is described, representing the area abutting the infraspinatus tendon. The supraspinatus and infraspinatus are intimately related and share many crossing fibres. Differentiation between them is less clear-cut than previously thought.
Leading-edge tears are those that reach and involve the portion of supraspinatus that lies adjacent to the biceps tendon/rotator interval. If there is intact tendon tissue between the biceps tendon and the tear, a midsubstance/crescent/footprint tear is diagnosed. What determines why a tear occurs in these different locations is not completely understood; however, the rotator cables described above may play a role.
Tears are occasionally further divided according to their shape, although this tends to be used more arthroscopically than with imaging. The simplest shape is linear. This is a separation from the humeral attachment, perpendicular to the normal direction of supraspinatus fibres. Linear tears may involve the anterior edge or midsubstance ( Figs 2.6 and 2.7 ). On ultrasound, these are much larger in one plane than the other. Extension in two tendon planes leads to the formation of L-shaped or tongue shaped tears ( Figs 2.8 and 2.9 ). When retraction occurs, the tears are described as V or U shaped ( Fig. 2.10 ).





Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree