Shoulder
Ian Beggs
INTRODUCTION
Ultrasound has become increasingly important in the assessment of the shoulder in recent years. It is as accurate as magnetic resonance imaging (MRI) in diagnosing rotator cuff tears1 and is also cheap and quick. Patients prefer ultrasound to MRI.2 The relative ease of access makes ultrasound ideal for a “one-stop shop” approach.3 Ultrasoundguided interventions are widely used. The objection that ultrasound is operator-dependent applies to other imaging techniques, clinical assessment, and surgical interventions.
ANATOMY AND EXAMINATION TECHNIQUE
The anatomy and examination technique of the shoulder is well illustrated on the website of the European Society of Musculoskeletal Radiology.4
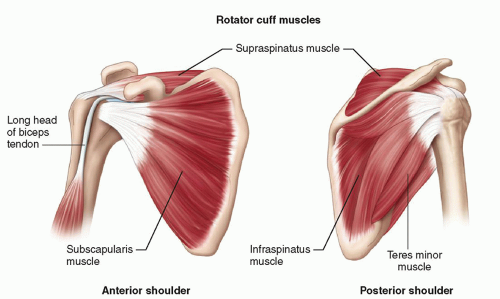
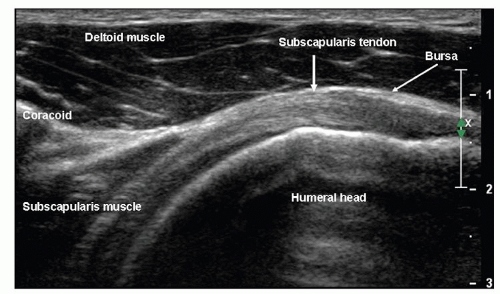
The tendons of the four muscles that contribute to the rotator cuff coalesce to insert on the greater and lesser tuberosities of the humerus (Fig. 3.1). Subscapularis originates from the anterior surface of the scapular blade and inserts on the lesser tuberosity. Supraspinatus originates from the suprascapular fossa and infraspinatus and teres minor from the dorsal surface of the scapula inferior to its spine. They insert in sequence from anterior to posterior on the greater tuberosity: supraspinatus on the superior facet and infraspinatus on the middle facet, although the footprint of the infraspinatus tendon is larger than that of supraspinatus and occupies much more of the tuberosity than previously thought. Teres minor inserts posterior to infraspinatus. Overlapping tendon fibers diffuse the load across the cuff rather than being concentrated in a single tendon.5,6 The subacromial/subdeltoid bursa is interposed between the rotator cuff tendons and the coracoacromial arch, which comprises the coracoid, acromion, coracoacromial ligament (CAL), and acromioclavicular joint (ACJ) (Fig. 3.2).
The long head of biceps (LHB) tendon is not part of the rotator cuff, but it is an important anatomical landmark. It originates from the superior glenoid labrum at the supraglenoid tubercle and runs through the joint,
separating subscapularis from the other rotator cuff tendons at the rotator interval (Fig. 3.1), then enters the bicipital groove and runs distally to its myotendinous junction in the mid-arm.
separating subscapularis from the other rotator cuff tendons at the rotator interval (Fig. 3.1), then enters the bicipital groove and runs distally to its myotendinous junction in the mid-arm.
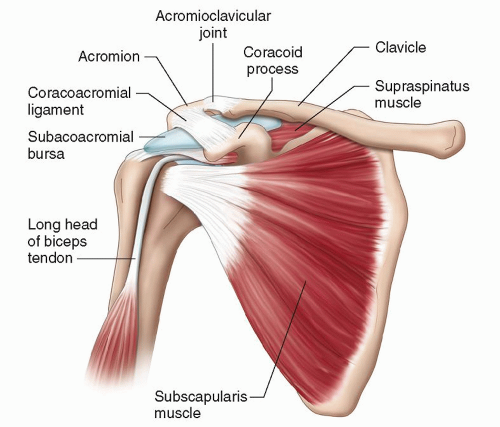 Figure 3.2. The subacromial bursa lies between the rotator cuff tendons and the coracoacromial arch. |
Supraspinatus and infraspinatus are innervated by the suprascapular nerve, a branch of the brachial plexus that passes through the suprascapular notch to supply supraspinatus and then through the spinoglenoid notch to supply infraspinatus. Subscapularis is supplied by subscapular branches of the brachial plexus and teres minor by the axillary nerve.
The articular cortex of the humeral head is usually smooth, although small pits are common. The greater and lesser tuberosities are usually slightly flat and depressed relative to the articular cortex. Hyaline cartilage covers the articular cortex. Enthesis fibrocartilage covers the tuberosities. Both types of cartilage are anechoic on ultrasound and appear in continuity.
ULTRASOUND EXAMINATION: TECHNIQUE AND APPEARANCES
In contrast to most other joints where the examination is targeted at a particular part of the joint to address a specific problem, ultrasound of the shoulder is usually a “whole joint” examination and is performed in a routine sequence.7,8
The patient is seated on a swivel chair and the height adjusted so that the patient’s shoulder is at a comfortable level for the examiner who stands in front of or behind the patient. I prefer to stand behind the patient. I can look over the patient at the ultrasound screen, lean forward to adjust the controls, and, importantly, support my hand on the patient’s shoulder. Standing in front of the patient places more stress on the examiner’s own shoulder.
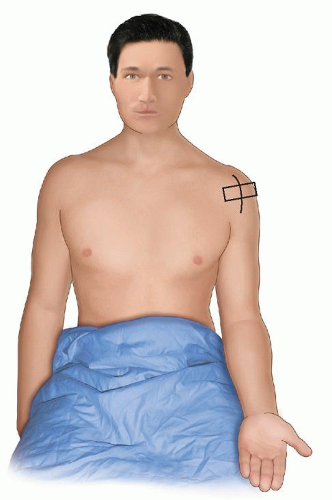
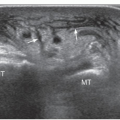
The examination starts with the patient’s elbow flexed at 90 degrees and the hand supine on the lap (Fig. 3.3). A transverse scan shows the biceps tendon as a round, well-defined, homogeneously echogenic structure in the bicipital groove (Fig. 3.4). A thin echogenic transverse “ligament,” probably actually fibers of the subscapularis tendon that continue from the lesser tuberosity to the greater tuberosity, covers the tendon at the entrance to the groove.
If the transducer is positioned slightly proximally, the biceps sling may be identified (Fig. 3.5). Moving the elbow posteriorly and rotating the transducer with the medial end slightly inferiorly may help. The sling is thought to be a more important stabiliser than the
transverse ligament. It is echogenic and has a horizontal U-shape with the base lying medially. The superior limb of the sling is the coracohumeral ligament (CHL), which merges with the anterior fibers of the supraspinatus tendon and also inserts on the greater tuberosity. The inferior limb is the superior glenohumeral ligament, which runs to the lesser tuberosity with some fibers of the CHL.9,10
transverse ligament. It is echogenic and has a horizontal U-shape with the base lying medially. The superior limb of the sling is the coracohumeral ligament (CHL), which merges with the anterior fibers of the supraspinatus tendon and also inserts on the greater tuberosity. The inferior limb is the superior glenohumeral ligament, which runs to the lesser tuberosity with some fibers of the CHL.9,10
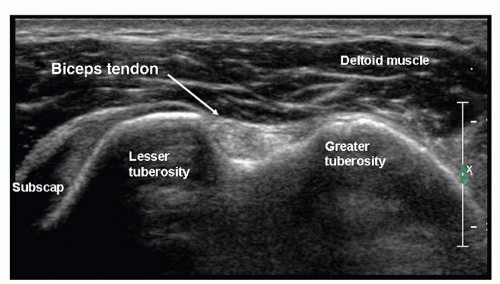 Figure 3.4. Biceps lies in the bicipital groove and appears round and echogenic on short-axis scans. |
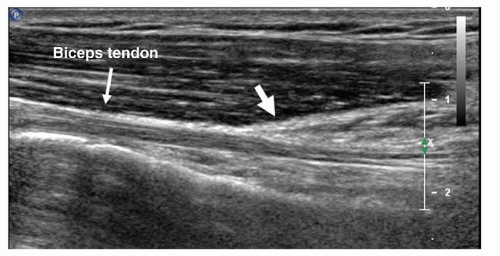
The full length of the extra-articular biceps tendon is easily examined in transverse images by an “elevator” technique, running the transducer proximally and distally between the bicipital groove and the musculotendinous junction of biceps, which lies level with the insertion of the pectoralis major tendon (Fig. 3.6), on the humeral shaft. The normal biceps tendon is well defined and homogeneously echogenic. Small amounts of fluid in the tendon sheath are normal. If the transducer is rotated 90 degrees biceps can be examined longitudinally (Fig. 3.7), but this is less useful than transverse scanning. On longitudinal scans the tendon appears cord-like and striated. Variable amounts of the intra-articular biceps can be seen, but ultrasound is not an appropriate examination for suspected proximal biceps lesions such as SLAP tears.
 Figure 3.6. Transverse scan of arm at the level of pectoralis major tendon (short arrows), which inserts on the anterior humerus alongside the myotendinous junction of LHB (long arrow). |
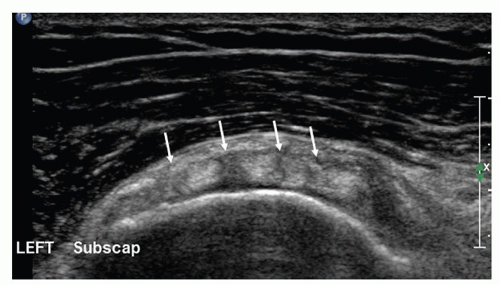
Next, to examine the subscapularis, the hand is externally rotated, still palm up with the elbow by the patient’s side and flexed at 90 degrees (Fig. 3.8). A longaxis scan of subscapularis (Fig. 3.9), with the transducer lying transversely on the anterior shoulder, shows the hypoechoic muscle emerging from under the coracoid process and running as echogenic tendon to insert on the lesser tuberosity. The distal 1 cm or so of the tendon may appear hypoechoic owing to anisotropy if the transducer is not angled “round the corner” of the tuberosity. On transverse images the tendon is echogenic and has curved superior and inferior margins. Alternating
hypoechoic and echogenic areas at the myotendinous junction (Fig. 3.10) are due to muscle interspersed between multiple tendon slips.
hypoechoic and echogenic areas at the myotendinous junction (Fig. 3.10) are due to muscle interspersed between multiple tendon slips.
Placing the transducer on the coracoid process and angling superiorly toward the acromion shows the CAL (Fig. 3.11). It is a thin, linear structure, small and oval on cross section.
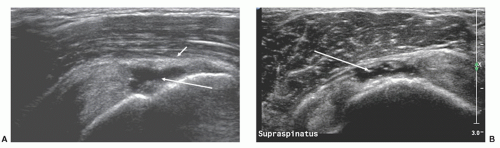
Supraspinatus is examined with the shoulder internally rotated and extended to pull the tendon out from under the acromion. Three positions may be used. First, the hand is placed behind the lower back with palm facing backward (Fig. 3.12). This may be too painful in patients with severe tendinosis or impingement, and in young patients may result in too much internal rotation to show the anterior edge of supraspinatus. The alternatives, which achieve progressively less internal rotation, are to use the “hand-in-back-pocket” position with the hand prone on the lateral buttock, or simply to have the arm hanging by the side with the palm facing backward.
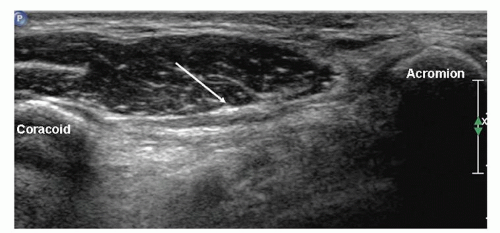 Figure 3.11. Coracoacromial ligament (arrow) is thin and broad and runs between the coracoid process and the acromion. |
Tip:
If you can’t see the rotator interval and anterior fibers of supraspinatus when the patient’s hand is behind his or her back, the shoulder is too internally rotated. Try the “hand-in-back-pocket” or “hand-by-side” positions.
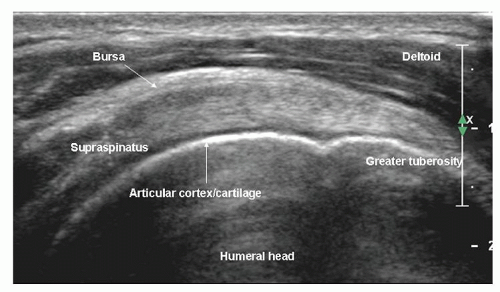
The transducer is placed parallel to the long axis or short axis of the tendon, equivalent to the coronal oblique and sagittal oblique views obtained with MRI. It is important to align the transducer to the axes of the tendon and not to body planes.
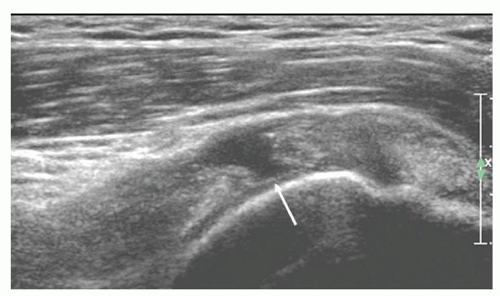
On long-axis views (Fig. 3.13) the tendon tapers smoothly toward its insertion on the greater tuberosity and has a convex superior surface. The thin, hypoechoic subacromial/subdeltoid bursa is superficial to supraspinatus and the other tendons of the cuff. Thin, parallel echogenic stripes of peribursal fat lie deep and superficial to the bursa. Fluid in the bursa can be an important clue to pathology, but may only be seen by scanning quite far distally, inferior to the tuberosities. Hypoechoic deltoid muscle overlies the bursa. There should therefore be three layers, deltoid, bursa/peribursal fat, and tendon, between the subcutaneous fat and the humeral head.
Tip:
Always identify three layers when examining supraspinatus: deltoid, bursa, and supraspinatus.
Supraspinatus is echogenic and has a uniformly striated appearance on long-axis images, but may appear hypoechoic if the angle of insonation is not 90 degrees. This occurs particularly distally where the tendon fibers swoop deeply to their insertion, and care must be taken to overcome the effect of anisotropy by altering the angle of the transducer and trying to “fill in” any hypoechoic areas.
Tip:
Try to “fill in” any hypoechoic areas in the rotator cuff by altering the angle of the transducer.
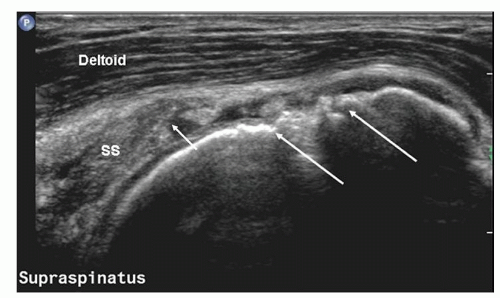
The entheseal fibrocartilage where the tendon inserts on the tuberosity is anechoic and appears virtually continuous with the hyaline cartilage on the articular cortex (Fig. 3.13), although a small bare area may be present at the junction between hyaline and entheseal cartilage. On short-axis images, supraspinatus has an echogenic speckled texture and is of uniform caliber, thinning progressively as it runs to its insertion. The anterior edge of supraspinatus normally overlaps the biceps tendon (Fig. 3.14).
The distal supraspinatus and infraspinatus tendons, which form the “rotator crescent,” are relatively hypovascular11 and are liable to injury. Occasionally, a small band of echogenic fibers, a few mm thick, runs transversely on the deep aspect of the tendon. This “rotator cable” appears to protect against tear propagation and reduces the functional disability caused by a rotator cuff tear.12
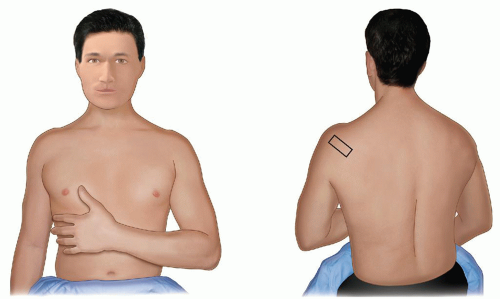
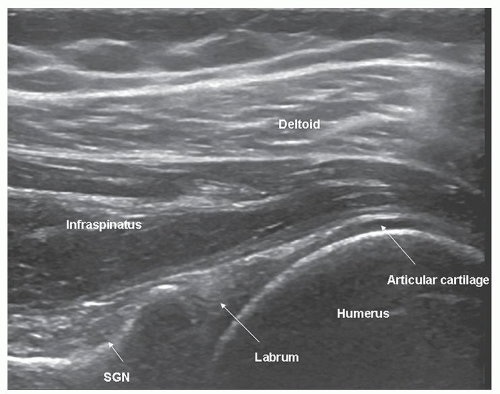
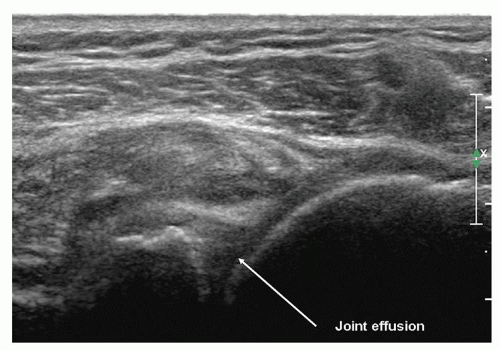
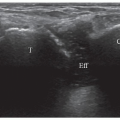
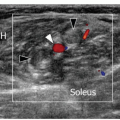
Infraspinatus is examined with the elbow flexed, the forearm across the chest, the patient’s hand palm down on the contralateral anterior chest wall, and the transducer placed transversely on the posterior aspect of the shoulder and angled slightly obliquely (Fig. 3.15). The hypoechoic infraspinatus muscle runs laterally and slopes gently superiorly. As it does so, the initially narrow intramuscular echogenic tendon widens to merge with the supraspinatus tendon and the hypoechoic muscle thins inversely. Deep to the infraspinatus (Fig. 3.16) is the posterior glenohumeral joint margin with the curved humeral head and the echogenic glenoid labrum. This is a good site to detect an effusion (Fig. 3.17), which elevates the capsule, or to inject into the joint. The spinoglenoid notch lies medially. A gangion due to internal derangement of the joint may extend to the notch and
compress the suprascapular nerve. The resulting infraspinatus weakness simulates a cuff tear.
compress the suprascapular nerve. The resulting infraspinatus weakness simulates a cuff tear.
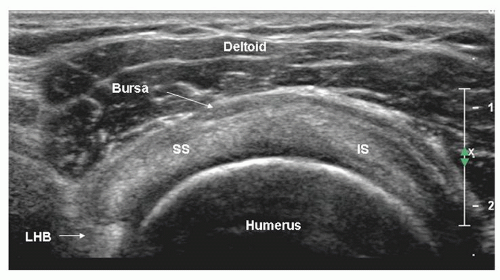 Figure 3.14. Short-axis scan of supraspinatus (SS) and infraspinatus (IS). The anterior edge of supraspinatus overlaps biceps (LHB). The bursa is thickened. |
Supraspinatus and infraspinatus form a continuous tendon sheet. Their junction may be recognised because of the slightly different orientations of the two tendons. It may be possible to identify the separate superior and middle facets of the greater tuberosity, the insertion sites of supraspinatus and infraspinatus, respectively. In practice, a distance of 1.5 cm behind the anterior edge of supraspinatus is said to mark the junction between the two tendons, although recent work suggests that infraspinatus may extend more anteriorly than this.5 If supraspinatus is torn, biceps is a good proxy for its anterior edge.
This concludes the standard examination, although some examiners routinely include the ACJ or dynamic assessment of impingement. Teres minor is not usually examined. Muscle integrity should be assessed if a fullthickness rotator cuff tear is identified.
ROTATOR CUFF TENDINOSIS AND TEARS
Complete thickness tears; partial thickness tears; tendinosis; accuracy; asymptomatic tears; muscle atrophy and fatty infiltration; impingement.
Management of the painful shoulder is based on the concept of rotator cuff impingement. Neer13 proposed that the interplay between the supraspinatus tendon and the coracoacromial arch, which includes the coracoid, anterior acromion, CAL, and ACJ, damages the tendon.
The distal anterior supraspinatus tendon is especially vulnerable because it is avascular.11,14 Initially, reversible damage is followed by irreversible tendinosis and fibrosis and finally by rotator cuff tears.
The distal anterior supraspinatus tendon is especially vulnerable because it is avascular.11,14 Initially, reversible damage is followed by irreversible tendinosis and fibrosis and finally by rotator cuff tears.
Clinical tests have only moderate sensitivity for rotator cuff tears15,16 and are subject to considerable interobserver variability.17 Experienced clinicians can accurately exclude rotator cuff tears, but depend on imaging to confirm if a tear is present.18
Full-thickness Rotator Cuff Tears
Most rotator cuff tears are said to start in the vulnerable zone in the anterior supraspinatus tendon close to the greater tuberosity of the humerus, while mid-substance tears that start more posteriorly are thought to be less frequent. However, recent work shows that most degenerate tears start close to the junction of the supraspinatus and infraspinatus tendons, about 15 mm posterior to the biceps tendon.19 As tears enlarge, they extend proximally and/or in anterior or posterior directions and may eventually involve all the rotator cuff tendons and the biceps tendon.
Full-thickness rotator cuff tears extend all the way from the superficial or bursal surface of the tendon to the deep or articular surface, but not necessarily across the full width from front to back, whereas partial-thickness tears extend only part way between bursal and articular surfaces.
A defect that extends from the bursal surface to the joint surface of the tendon is the primary ultrasound sign of a full-thickness tear, and it should be present on both long-axis and short-axis scans of the tendon (Fig. 3.18). If an apparent defect is identified, the transducer should be angled to try to “fill in” the defect in case the appearance is due to anisotropy, and the defect should be shown on both longitudinal and transverse images. If the tear is very large and the tendon retracted under the acromion, no tendon is visible.
Tip:
Confirm a tear is present by showing a defect on both longaxis and short-axis images.
Fluid-filled defects are easy to identify because of the contrast differences between the fluid and the edges of the torn tendon, which may be slightly depressed. Tears are more difficult to identify if the fluid is echogenic or the defect occupied by synovium, debris, or by deltoid muscle (Fig. 3.19) that has herniated into the defect. Small tears can produce focal thinning or bursal surface depression indistinguishable from partial-thickness tears or severe tendinosis. Repeatedly pressing on the tendon with the transducer may move fluid or debris and make a small tear more obvious or distinguish a complete-thickness tear from a partial-thickness tear.
Tip:
Sonopalpation may make a tear more conspicuous or distinguish between full-thickness and partial-thickness tears.
Tear margins may be well defined or irregular. Delamination results in hypoechoic clefts that run transversely from the tear edge into the tendon. Rarely such a cleft communicates with a fluid-filled intramuscular cyst.
Tear extension >15 mm posterior to the anterior edge of supraspinatus tendon, which in practice is defined by the biceps tendon, indicates that the infraspinatus tendon is also torn. This measurement technique cannot be used if biceps is also torn, but the combination of biceps and supraspinatus tears usually indicates that the cuff tear is very large.
Tip:
Calculate supraspinatus tear width by measuring from the biceps tendon.
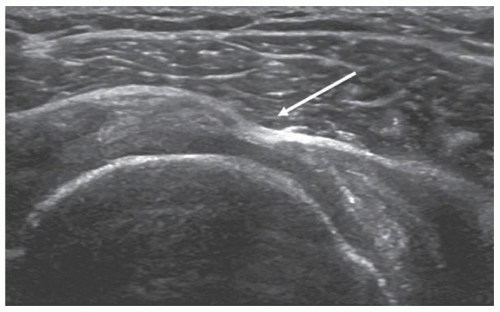 Figure 3.19. Short-axis scan of narrow full-thickness supraspinatus tear (arrow) with herniation of deltoid into defect. |
Large tears result in tendon retraction under the acromion (Fig. 3.20), when the proximal edge of the tear cannot be seen. The deltoid muscle may herniate into the defect and be in direct contact with the humerus. It is important to recognise that there is a missing layer. Proximal migration of the humeral head to be in direct contact with the acromion (Fig. 3.21) occurs with very large chronic tears.
Full-thickness tears are associated with synovial inflammation20 and abnormal signal is occasionally seen on Doppler ultrasound, but this is not a regular feature.
If a full-thickness tear is identified, the supraspinatus and infraspinatus muscles should be assessed for atrophy and fatty infiltration.
Secondary signs are highly suggestive of full-thickness tears but are not diagnostic. Depression or flattening of the bursal surface (Fig. 3.22) is a powerful indicator of pathology. It is nonspecific and occurs in both tears and tendinosis, although the deeper the depression, the more likely a full-thickness tear.
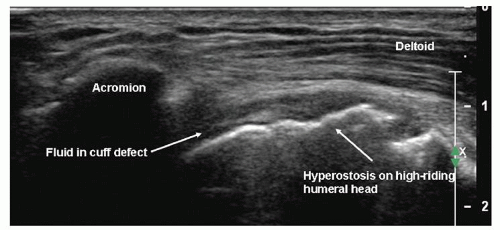 Figure 3.21. Massive rotator cuff tear. The cuff is retracted under the acromion, and the humeral head is high-riding. |
Tip:
Bursal surface flattening or depression are sensitive indicators of cuff pathology, but are nonspecific.
The combination of glenohumeral joint fluid and hyperostosis at the greater tuberosity (Fig. 3.20) has a positive predictive value (PPV) and specificity of 100% for full-thickness tears. Fluid in the subdeltoid bursa and joint has a PPV of 95% and specificity of 99%. The bursa extends distal to the greater tuberosity (Fig. 3.23), and sometimes fluid is seen only in the distal segment. My experience is that fluid in the bursa and biceps tendon sheath also strongly suggests a tear, although a PPV of only 54% has been reported. Hyperostosis at the greater tuberosity or fluid in any one of the joint, bursa or biceps tendon sheath are less useful, having PPV value of 60% to 70%. A smooth tuberosity is likely to be associated with an intact tendon.21,22,23,24
The cartilage interface sign (Fig. 3.24) is an echogenic line at the surface of the hyaline cartilage on the humeral head deep to a cuff tear, and is due to reduced attenuation and altered acoustic interface. I do not find this a useful sign. The surface of the articular cartilage is often quite echogenic, especially with modern equipment, and
hyperechoity is subjective. In any case, the sign is frequently most conspicuous when there is an obvious tear and fluid is present.
hyperechoity is subjective. In any case, the sign is frequently most conspicuous when there is an obvious tear and fluid is present.
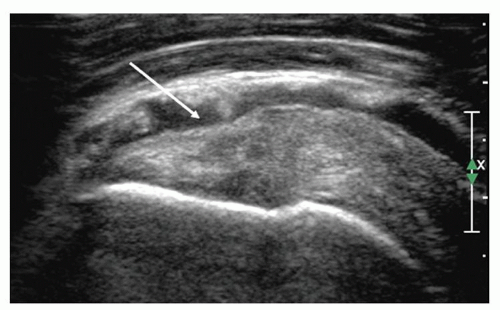 Figure 3.22. The effusion in the subdeltoid bursa shows that the bursal surface of supraspinatus is depressed (arrow). |
Tip:
These two combinations have high PPV for full-thickness rotator cuff tears:
Hyperostosis at the greater tuberosity and fluid in the glenohumeral joint.
2. Fluid in the glenohumeral joint/biceps tendon sheath and subdeltoid bursa.
Partial-Thickness Rotator Cuff Tears
Partial-thickness tears extend part way between the bursal and the articular surfaces of the cuff and may be at the surface or intratendinous. Joint side tears are most common.25
Bursal-side tears cause defects or depressions (Fig. 3.22) in the bursal surface of the cuff, usually near the greater tuberosity. The bursa may dip into the defect and may be conspicuous because of intrabursal fluid or echogenic peribursal fat. Intrasubstance tears are hypoechoic. A longitudinal split in the tendon may be identified. Articular-side tears (Fig. 3.25) are easily identified if the defect is fluid-filled, but are frequently quite difficult to identify and may have a mixed hypoechoic and hyperechoic appearance. Rim-rent tears (Fig. 3.26) are partialsubstance tears that are minimally retracted from the greater tuberosity and characteristically have a central echogenic “streak” surrounded by hypoechoic fluid.26,27
Rotator Cuff Tendinosis or Tendinopathy
Tendinopathy initially results in swelling and altered echogenicity. Swelling can be confirmed by comparing the affected segment with adjacent tendon or with the same position at the opposite shoulder. The difference in measurement is usually <2.5 mm. Thickness of >8 mm is also considered abnormal.28
Subsequently, the tendon may become thinned. Alterations in texture (Figs. 3.27 and 3.28) include reduced
echogenicity, mixed hypoechoity and hyperechoity, and foci of microcalcifications (Fig. 3.29), although heterogeneity is often seen in older patients without clinical evidence of tendinosis. Thickening of the subdeltoid bursa and small amounts of fluid in the bursa may be present, and there may be subtle loss of definition between tendon and bursa.24,28,29,30,31
echogenicity, mixed hypoechoity and hyperechoity, and foci of microcalcifications (Fig. 3.29), although heterogeneity is often seen in older patients without clinical evidence of tendinosis. Thickening of the subdeltoid bursa and small amounts of fluid in the bursa may be present, and there may be subtle loss of definition between tendon and bursa.24,28,29,30,31
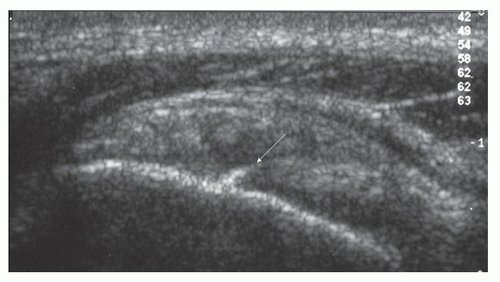 Figure 3.26. Echogenic streak (arrow) surrounded by hypoechoic fluid in supraspinatus adjacent to greater tuberosity typical of a “rim rent.” |
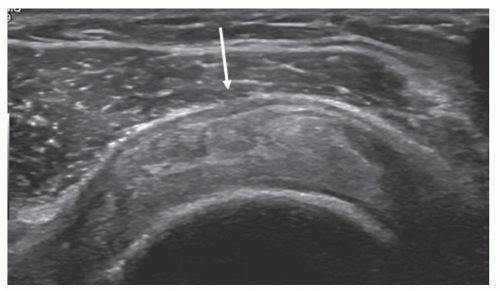 Figure 3.27. Supraspinatus tendinosis: the arrow points to bursal thickening, depression of the bursal surface of the tendon, and heterogeneous tendon texture. |
Neer’s theory postulates that increasingly severe grades of tendinosis develop into partial-thickness tears and then into full-thickness tears. Just as there are theoretical overlaps, there are overlaps between the ultrasound appearances of tendinosis and partial-thickness tears and between partial-thickness and full-thickness tears.
Accuracy of Ultrasound Detection of Rotator Cuff Tears
Ultrasound of the shoulder has been criticised as inaccurate and operator-dependent. Some initial reports recorded poor results. Subsequent studies have shown that there are no statistically significant differences between ultrasound and MRI in the detection of rotator cuff tears or the assessment of tear size compared with surgical results.32,33,34 Performing MRI after normal ultrasound of the rotator cuff confers no benefit.35
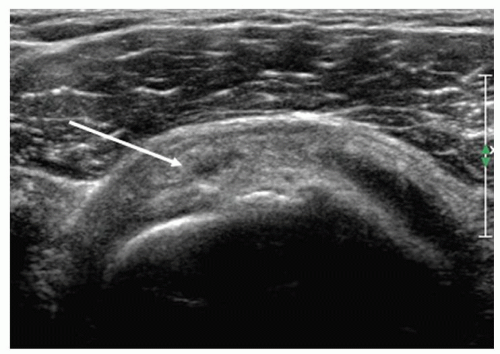 Figure 3.28. Supraspinatus tendinosis: the arrow points to an intrasubstance tear. The adjacent tendon is heterogeneous, and there is bursal surface flattening. |
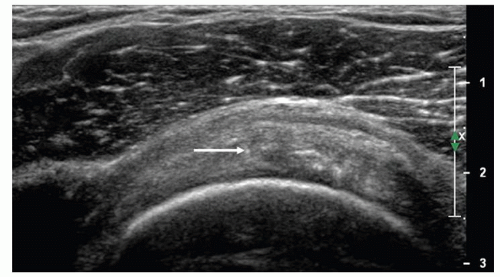 Figure 3.29. Short-axis scan of supraspinatus showing tendinosis. Microcalcification (arrow) and heterogeneous tendon texture are present. |
A series of meta-analyses1,18,36 has confirmed that ultrasound and MRI are equally accurate in the detection of rotator cuff tears, although results for both modalities are poorer for partial-thickness tears than full-thickness tears, and MR arthrography is a more accurate technique by a very small margin (Table 3.1).
Ultrasound errors include simple misses, usually of very small (<5 mm) tears, miscategorisation between fullthickness and partial-thickness tears or between partialthickness tears and tendinosis, and when patients are too big (heavily muscled or obese) or have such a restricted range of movement that parts of the rotator cuff are inaccessible.37
TABLE 3.1. Accuracy of Imaging in Rotator Cuff Tears | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree