and Marguerite M. Caré1
(1)
Department of Radiology ML5031, Cincinnati Children’s Hospital, Cincinnati, Ohio, USA
Keywords
Subdural hemorrhageHypoxic-ischemic injuryAbusive head injury6.1 The Skull
Linear, parietal bone fractures are the most common fracture type and location for pediatric skull fractures resulting from both accidental and abusive head injuries [1, 2]. Fractures caused by abuse represent the minority of the skull fractures seen in the overall pediatric population [3], many occurring from short to moderate distance falls both in and out of the home. However, when looking at infants and young children less than 12–24 months, abusive fractures constitute a larger percentage of the identified fractures [1, 2]. Although short distance falls may result in a skull fracture, they are usually neurologically benign [4] with minimal to no intracranial injury or hemorrhage. However, the report of a short fall is a frequent history provided in some cases of abusive injury, lending confusion to the investigation.
Since skull fractures are seen frequently in pediatric patients, are certain patterns of injury present that might be more concerning and suggestive of abuse? Hobbs [5] retrospectively reviewed 89 infants and young children less than 2 years presenting with skull fractures, 29 that were considered definite abusive injury. In their study cohort, 20 of the infants died, 19 of those cases were in the abused group, suggesting that many of these infants likely represented the more severe end of the spectrum of pediatric skull fractures that might not be seen in a more routine pediatric practice. However, this study concluded that complex or multiple fractures, diastatic or depressed fractures, growing fractures, fractures involving more than one bone or non-parietal fractures, and those associated with an intracranial injury were seen more frequently in the abusive head injury cases.
In a review of both accidental and abused infants and young children, Meservy et al. [1] found that multiple, bilateral, and fractures that crossed cranial sutures occurred more frequently in the children that were abused. However, depressed and diastatic fractures, complex, and fractures occurring in a non-parietal location were not found to be significantly different between the two groups. This population was less biased toward severe abusive cases and likely represents a more typical pediatric patient population. More recently, Barber et al. [6], looking at a population of infants with suspected abuse undergoing skeletal surveys, found complex skull fractures in 45 % of the infants presenting with skull fractures on the initial skeletal survey. However, linear fractures were found more commonly in their population, occurring in 55 % of cases, but those with a complex fracture pattern were significantly more likely to have other fractures detected on skeletal survey, thus raising concerns for abusive injury.
Leventhal et al. [7], using a national database, looked at children less than 36 months to determine abusive versus accidental skull fractures with or without associated traumatic intracranial injury. In children that were found to have only a skull fracture , almost all of the injuries occurred by accidental mechanisms, especially in children less than 2 months. This study also found that in children with skull fractures and traumatic intracranial injury, accidental mechanisms, such as falls, were still more likely. However, in young children and infants less than 12 months of age with skull fractures, traumatic brain injury and skeletal fractures other than the cranium or with nonskull fractures and traumatic brain injury, almost all of these injuries were the result of abuse.
Although skull radiographs or images are still obtained as part of the skeletal survey that is used in the evaluation of potential abusive cases [8, 9], skull radiographs are less commonly being used in the routine evaluation of children and infants that present with reported minor trauma [8], as imaging, if it is performed at all, has shifted towards CT. However, with concerns of potential radiation effects, knowing when to image is not always straightforward, except in high-risk cases. Pediatric guidelines exist [10], but physician experience and local guidelines for imaging patients with concerns of potential abuse must also be utilized. Some authors have suggested using rapid, non-sedated MRI in potential trauma cases [11, 12], however, at our institution, we continue to use CT, as the study is quick, readily available, and excellent at detecting both cranial and intracranial injuries. MRI, although often excellent at detecting intracranial injuries, has been shown to be much less sensitive at detecting skull fractures [13].
Whether from accidental or abusive injuries, our ability to detect subtle fractures, many that might have been difficult to see during the era when only axial CT images could be obtained, has clearly increased with the routine use of sagittal, coronal, and 3-dimensional reconstructions of the skull from CT head exams (Fig. 6.1). Radiology practices should consider routinely including these multiplanar reconstructions, especially in the pediatric and child abuse populations where they also help delineate normal structures, such as sutures or vascular grooves from a potentially overcalled fracture [14] (Fig. 6.2). These reconstructions further delineate depressed or complex fractures of the skull and may help to show how a fracture that appears more complex on radiographs may actually represent one impact, as the fracture extent is more easily followed across sutures or over the cranial vertex. At our institution, if a child has a head CT with multiplanar reconstructions prior to the skeletal survey, we eliminate the skull radiographs from the initial skeletal survey, as we have found that no skull fracture detected on skull radiographs was missed on the head CT images.
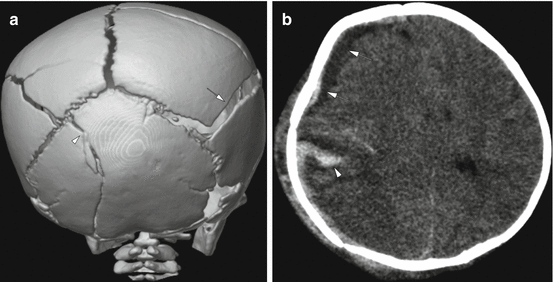
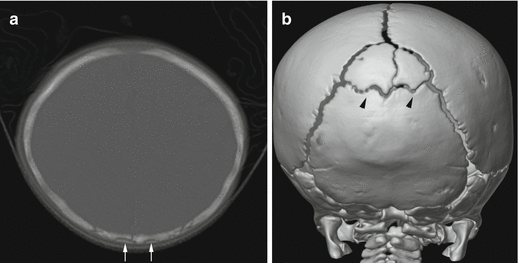

Fig. 6.1
Complex skull fractures in a 9-week old infant. (a) 3-dimensional reconstruction of the posterior skull demonstrates complex fractures involving both parietal bones, more diastatic on the right (arrow). There is also extension into the left occipital bone (arrowhead). (b) There is a mixed attenuation subdural hemorrhage (arrows) with mass effect and midline shift, as well as a suspected laceration of the brain (arrowhead). The child subsequently underwent an emergent decompressive hemicraniectomy

Fig. 6.2
3-month old with bruising and metaphyseal fractures. (a) Axial CT shows a transversely oriented lucency (arrows) in the occipital bone. (b) This is more easily confirmed to be intrasutural bones (arrowheads) on the 3-dimensional reconstructions of the skull
The evaluation of soft tissue structures is also important in the evaluation of the skull. Subtle, asymmetric soft tissue swelling may lead the radiologist to more carefully evaluate the underlying bones and allow detection of subtle fractures. Acute fractures usually present with overlying soft tissue swelling, but swelling may be difficult to detect clinically in some patients. It is often helpful to review the bone algorithm images on a more intermediate setting, such as would be used to view an abdomen CT, to look for subtle swelling. In some cases, soft tissue swelling may be difficult to detect initially and may increase over a period of days. Also, lack of swelling may point to a more remote fracture.
Unlike long bone injuries, skull fractures do not demonstrate the typical pattern of healing and periosteal reaction that is used to detect skeletal injuries on follow-up skeletal surveys. Therefore, skull radiographs should not be routinely included on follow-up skeletal surveys looking for fractures that may have been occult on the initial exam [15]. Gradually, skull fractures become less distinct over a period of several months and are usually completely healed by 4–6 months.
6.2 Benign Macrocrania
Benign macrocrania , also called benign extra-axial fluid or benign enlargement of the subarachnoid spaces (BESS), is a common clinical and imaging finding in young infants. Many of these infants present to the radiology department with the history of macrocephaly or an enlarging head circumference. Imaging may be performed with MRI or CT, but often these infants undergo a head ultrasound through the open anterior fontanel. Benign macrocrania frequently presents around 3–6 months of age and often resolves by 24–36 months. These enlarged spaces do not result in developmental delays.
Infants, in general, typically have larger extra-axial fluid spaces than older children. In infants with benign macrocrania, the subarachnoid fluid spaces are even more prominent than normal. On imaging, the ventricles are frequently upper normal in size to mildly enlarged, but they may be normal. Cortical and bridging veins are seen coursing through the enlarged subarachnoid spaces, a finding most easily appreciated on ultrasound or MRI (Fig. 6.3), as opposed to displacement of the vessels in a child with subdural collections or hemorrhage (Fig. 6.4). However, with the location of prominent subarachnoid spaces typically in a bi-frontal distribution and causing slight widening of the adjacent sulci, as well as relying on multiplanar reconstructions, CT imaging can often distinguish the enlarged subarachnoid spaces from low attenuation, abnormal subdural collections.
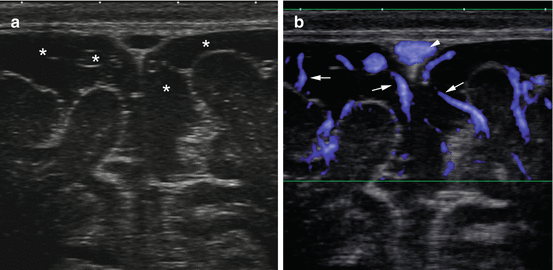
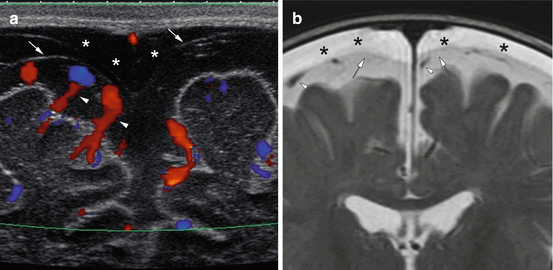

Fig. 6.3
9-month old with macrocephaly. (a) Gray-scale ultrasound image showing enlarged subarachnoid spaces (asterisks). (b) Coronal Doppler US shows mild enlargement of the subarachnoid spaces with the bridging vessels (arrows) coursing within the subarachnoid space and extending toward the sagittal sinus (arrowhead)

Fig. 6.4
2-month old with macrocephaly shows bilateral subdural collections (asterisks) with visualization of the arachnoid membrane (arrows) and vessels (arrowheads) within the subarachnoid space on US (a) and MRI (b)
Recent investigations [16, 17] looking at infants with macrocrania and enlarged extra-axial spaces have found about 4–5 % of these infants may have very small, not overtly hemorrhagic subdural collections. These are thin, usually present in a frontal distribution, and do not cause mass effect on the brain or acute decompensation of the infant. The radiologist needs to consider the size and location of these collections, and if fluid signal or near-fluid signal, may conclude that these collections may not be related to prior head injury. However, it might be prudent to discuss these cases with the referring physicians so that any clinical concerns of abuse might be addressed.
Although thin subdural collections may occur in the setting of enlarged extra-axial spaces, other manifestations of abusive head injury, such as large, multifocal subdural hemorrhage , parenchymal injury, and retinal hemorrhages are not explained. As many children have larger extra-axial spaces, concern for abusive head injury should be present with more clearly hemorrhagic collections, especially when these other injuries coexist.
6.3 Subdural Hemorrhage
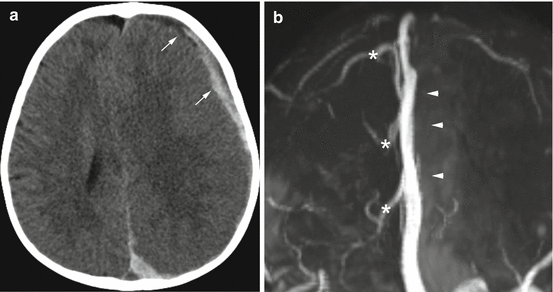
Subdural hemorrhage is the most frequent imaging finding following abusive head injury [18–20]. Not infrequently, it is seen in combination with other signs of more significant trauma including subarachnoid hemorrhage , brain injury, and retinal hemorrhages. Classically, the subdural bleeding is thought to occur from injury or tearing of bridging veins that extend from the surface of the brain to the dural venous sinuses with disruption of the dural-arachnoid junction [21] and accumulation of hemorrhage in this “potential space or compartment”. In the young child, bridging vein injury may be recognized in the operating room in cases that require emergent surgical decompression, at autopsy [21], as well as on imaging [22] with small tubular or spherical regions of clot at the site of vein injury (Fig. 6.5).
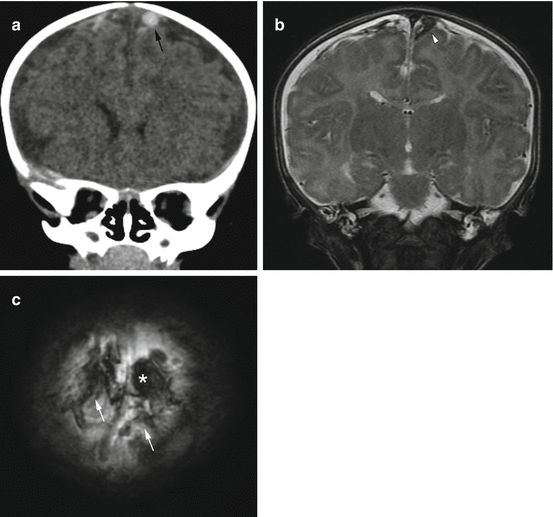

Fig. 6.5
Focal clot at the site of bridging vein injury in a 2-month old with multifocal subdural hemorrhages and extensive, bilateral retinal hemorrhages. (a) Coronal CT reconstruction shows focal, rounded clot at the site of an injured bridging vein (black arrow). This has been termed the “lollipop sign” by some authors [22]. (b) Corresponding T2-weighted MR image on day 2 again shows the focal clot (arrowhead) with adjacent vein. (c) Axial susceptibility image shows blooming at the sight of the focal clot (asterisk), as well as areas of hemorrhagic staining along several bridging veins (white arrows)
Traumatic subdural hemorrhage is not unique to abuse and may also result from accidental injury. However, subdural or extra-axial hemorrhage that occurs as a result of a minor accidental injury or a short fall usually results in hemorrhage that is more localized to the site of injury or impact. More significant accidental injuries, such as motor vehicle injuries or children hit by cars, may result in more diffuse and multifocal subdural and subarachnoid hemorrhage, as well as craniocervical and brain injuries. With abusive head injury, the subdural hemorrhage may be bilateral, although it is frequently asymmetric and may extend along a single hemisphere. It is often multifocal, heterogeneous in attenuation, found high over the cerebral hemispheres, and present along the interhemispheric region and tentorium (Fig. 6.6). In abusive cases, subdural hemorrhage is frequently also found in the posterior fossa, along the clivus [23], and in the spinal canal. Spinal subdural hemorrhage has been shown to be a more frequent finding in patients with inflicted injuries [24] compared to a cohort of accidentally injured infants and young children. In many cases of abusive injuries, the size of the traumatic lesion or hemorrhage is thin and less extensive compared to the degree of hypoxic-ischemic injury to the brain [25, 26].
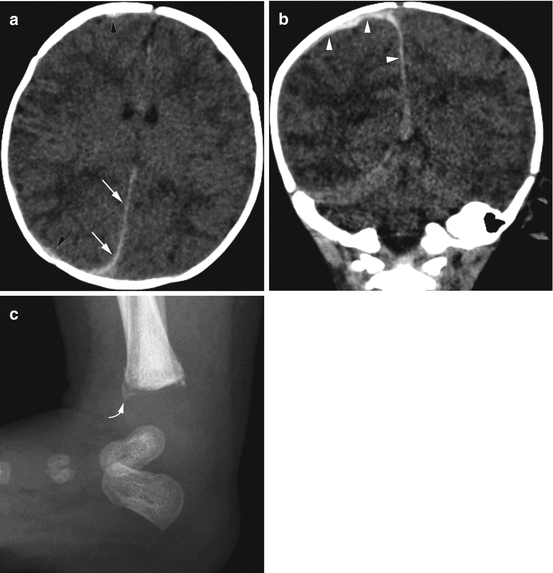

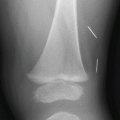
Fig. 6.6
Acute subdural hemorrhage in a 9-week old with facial bruising and fussiness. (a) Axial CT image demonstrates subtle high attenuation causing thickening along the posterior falx (white arrows) and adjacent to the right frontal and parietal lobes (black arrows). (b) Coronal CT reconstruction more clearly shows the high attenuation, acute subdural hemorrhage (arrowheads). (c) Lateral ankle image shows a metaphyseal injury (curved arrow) with subtle early healing
In the very young infant, it is important to distinguish potential birth-related hemorrhage from hemorrhage related to inflicted injury. Subdural hemorrhage may occur following both vaginal and cesarean deliveries. In these situations, it is frequently thin (less than 3 mm) and posterior in location, but it may be both supratentorial and infratentorial in location and usually causes very little mass effect, if at all. These thin hemorrhages are usually asymptomatic and almost all have been shown to resolve by about 1 month of age [27, 28]. Certainly, in more traumatic deliveries, larger and more symptomatic hemorrhage may occur.
Hematologic and vascular etiologies rarely result in subdural hemorrhage or hemorrhagic collections in the pediatric population, but these may include coagulopathies such as Neonatal Alloimmune Thrombocytopenia [29] or Vitamin K Deficiency [30]. Metabolic etiologies are often discussed as potential etiologies, but clinically, are rarely seen. More frequently, subdural collections may occur in patients following shunting procedures, in post-operative patients, or in children with significant brain volume loss. Infectious processes may result in subdural empyemas or effusions, but these patients are usually not clinically confused with potential abuse victims.
Much debate has been centered on subdural hemorrhage over the past many years [31, 32]. Although sometimes met with hostility, this debate has been useful, and it has reaffirmed the importance of a multidisciplinary approach in the workup of a young patient with suspected abuse. It has also caused radiologists to critically review the importance of not making blanket statements about the dating or aging of subdural hemorrhage based on the CT and MRI characteristics, something we are all taught and learn during our training [33, 34]. Physicians interpreting imaging should appreciate the variations in how subdural hemorrhage may look, even acutely, as well as how different imaging factors may create confusion and difficulty with timing (Fig. 6.7). Serial imaging with a description and identification of how the hemorrhage changes in size, location, and attenuation or signal is more helpful in trying to provide a time-frame for potential injury. Also, correlating the change in brain injury over serial scans in invaluable and should also incorporate the clinical/historical knowledge of the patient [18]. However, in some cases, only a single imaging study may be available, and review of the imaging to look for sometimes subtle subdural hemorrhage which remains an important “marker” of potential abuse may be key.
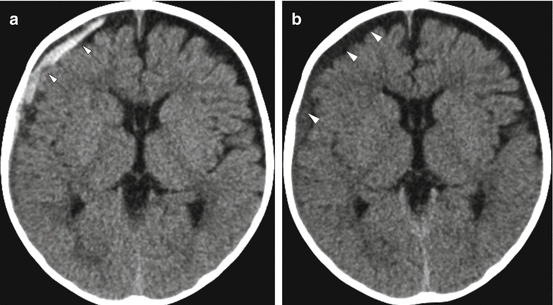

Fig. 6.7
Evolution of subdural hemorrhage in a 2-month old with a reported fall off of a couch. (a) Initial head CT demonstrates a mixed attenuation hemorrhagic subdural (arrows) without mass effect on the right cerebral hemisphere. (b) A CT obtained on day 2 now demonstrates a more homogeneous right subdural collection, likely representing evolution and mixing of the blood with cerebrospinal fluid (arrowheads) or fluid leakage related to a dural-arachnoid injury
One controversy questioning the etiology of subdural hemorrhage has centered on whether spontaneous intracranial venous thrombosis may be the cause of the subdural hemorrhage that is seen in cases of suspected abuse rather than the result of a traumatic injury. However, venous thrombosis and venous injuries are well known associations occurring following head trauma [22, 35], including abusive head trauma. Choudhary et al. [22] found signs of direct venous injury in 44 % of the children they studied with diagnosed abusive head injury that had undergone brain MR imaging and MR venography. In these cases, the direct venous injury is thought to be the cause of the subdural hemorrhage. Isolated subdural hemorrhage has not been an imaging finding previously reported in studies looking at the imaging findings in patients and children with cerebral venous thrombosis [36, 37]. McLean et al. looking at 36 infants and children with nontraumatic causes of intracranial venous thrombosis undergoing CT and MR imaging found no patients with associated subdural hemorrhage, even in one patient with extensive thrombosis and several with multifocal regions of thrombus. Spontaneous venous thrombosis is not the cause, but rather a result of a traumatic event that results in the subdural hemorrhage [22, 37]. However, to be prudent, routine evaluation of the venous sinuses and cortical veins, either by MR venography or post-contrast volumetric MR imaging should be considered in imaging protocols in patients with suspected abuse (Fig. 6.8).