Although the visualization of normal bronchioles is impaired by the spatial resolution limits of the thin-section CT, these airways may become directly visible when inflammation of the bronchiolar wall and accompanying exudate develop. On the other hand, bronchiolar changes can be too small to be visible directly but can cause indirect signs that suggest small airway involvement. Obstruction of the bronchioles may induce regional underventilation, leading to reflex vasoconstriction and expiratory air trapping, both of which may be visible on CT images. Five different CT patterns can express small airway pathology. The first two are direct signs, and the other three represent indirect manifestations.
Tree-in-Bud Sign
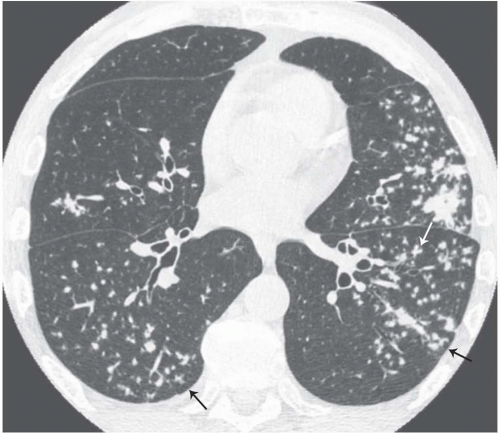
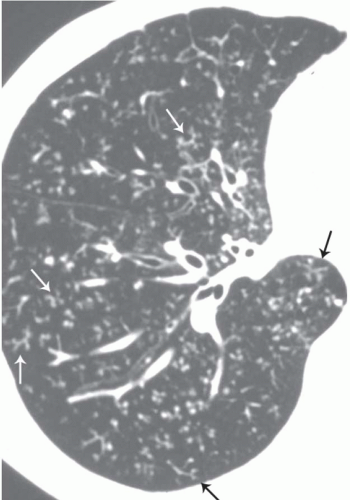
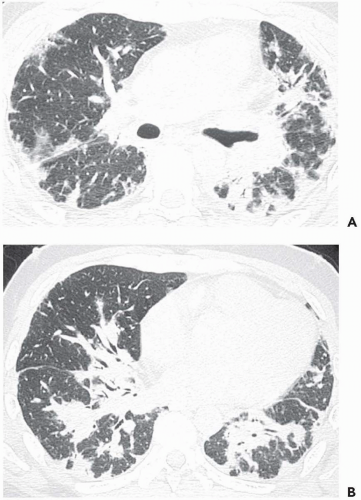
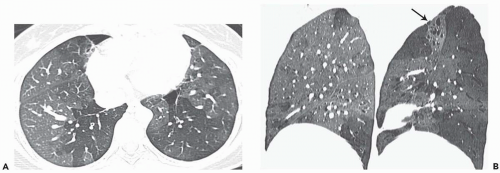
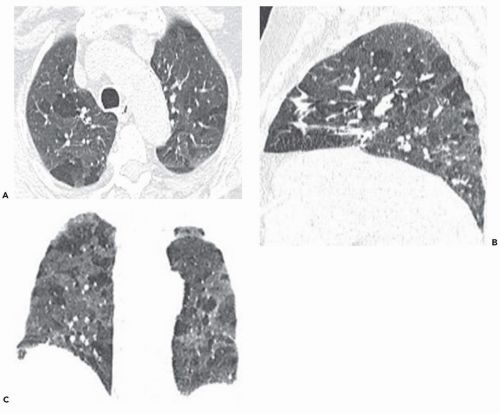
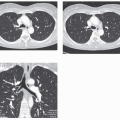
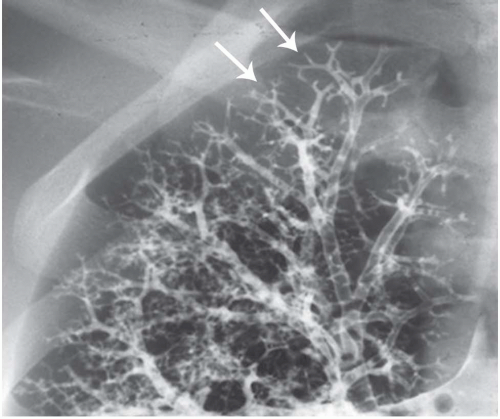
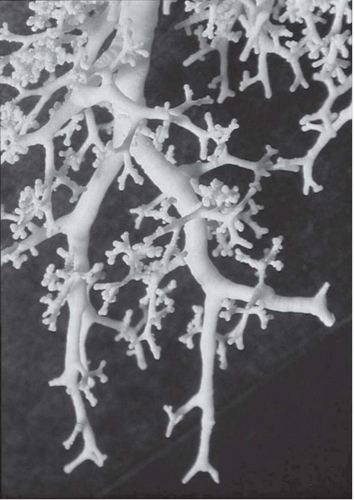
The tree-in-bud pattern comprises focal or multifocal areas of small centrilobular nodular and branching linear opacities. It reflects the abnormal bronchiolar wall thickening and dilatation of the bronchiolar lumen filled with liquid, mucus, or pus that is often associated with peribronchiolar inflammation (
7). The branching pattern of dilated bronchioles and peribronchiolar inflammation give the appearance of a budding tree (
Fig. 5-8). Some variants have the same diagnostic value. They include clusters of centrilobular nodules linked together by fine linear opacities or branching tubular or Y-shaped opacities without nodules (
Figs. 5-9 and
5-10). When the bronchiole is sectioned across its axis the filled dilated lumen may appear as a single well-defined
centrilobular nodule a few millimeters in diameter. In every case, the key feature is the centrilobular location of these opacities, at least 3 mm from the pleura.
The tree-in-bud sign is characteristic of acute or chronic infectious bronchiolitis (
34). It can also be seen in diffuse panbronchiolitis, diffuse aspiration bronchiolitis, and bronchiolitis obliterans with intraluminal polyps featuring bronchiolar filling with granulation tissue (
35).
The tree-in-bud sign is distinguished from abnormal centrilobular perivascular interstitial thickening by its more irregular appearance, a lack of tapering, and the bulbous or knobby appearance of the tips of small branches. Some difficulties in interpretation may occur. Bronchiolar filling with tumor cells, observed in bronchioloalveolar cell carcinoma or tumor emboli within small centrilobular arteries, can produce centrilobular nodules and branching lines (
36,
37,
38,
39). In the same way, peribronchiolar nodules resulting from sarcoidosis that occur in relation to the centrilobular arteries occasionally may mimic the appearance of the tree-in-bud sign (
7,
35). Hopefully, other typical features of sarcoidosis usually also are present.
The frequent association of tree-in-bud sign with other findings of airway disease facilitates the diagnosis of small airway disease. They include areas of air-filled bronchiolar dilatation; ill-defined centrilobular nodules, reflecting areas of inflammation; bronchiolar wall thickening; and bronchiectasis.
Poorly Defined Centrilobular Nodules
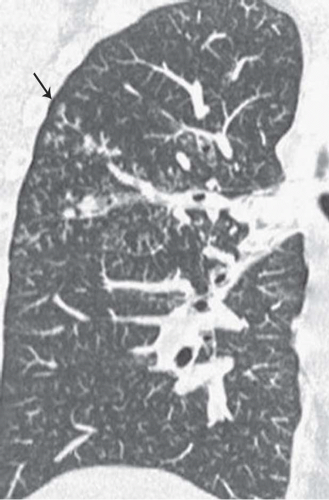
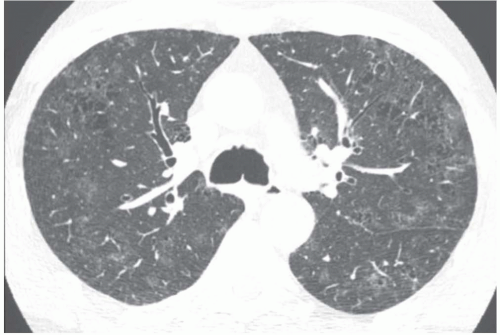
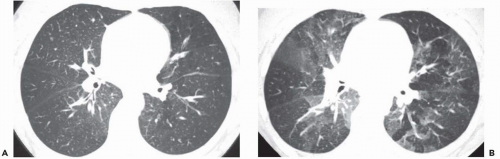
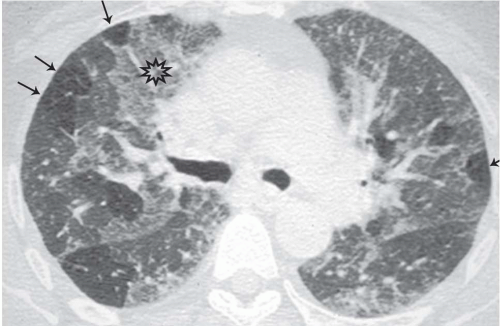
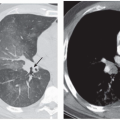
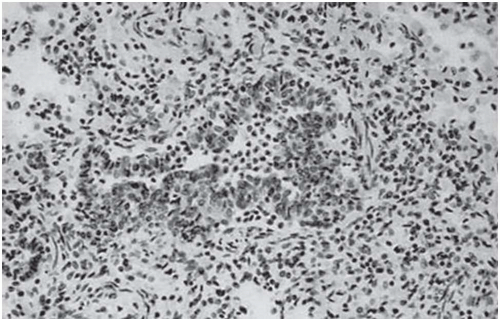
The presence of ill-defined centrilobular nodules in patients who have bronchiolar disease usually reflects the presence of peribronchiolar inflammation in the absence of airway filling with secretion (
40) (
Fig. 5-11). Unfortunately this pattern is not specific to small airway disease and may also suggest a vascular or interstitial disease (
41). When the finding is patchy in distribution, the list of entities potentially responsible is wide, including inflammatory/infectious bronchiolitis, asbestosis, silicosis, Langerhans cell histiocytosis,
vasculitis, pulmonary hypertension, sarcoidosis, or, rarely, BOOP. When the distribution of nodules is diffuse and homogeneous, the pattern is suggestive of bronchiolar disease or vascular entities, including pulmonary edema, pulmonary hemorrhage, and capillary hemangiomatosis. Differential diagnosis with bronchiolar disease is based on associated findings, such as pleural effusion in edema and enlargement of proximal pulmonary arteries. Bronchiolar possibilities include respiratory bronchiolitis, bronchiolitis associated with hypersensitivity pneumonitis, and follicular bronchiolitis (
7,
40). Despite the large number of diseases potentially responsible for this pattern, in most cases the differential diagnosis is simplified by detailed clinical information, including occupational and environmental histories.
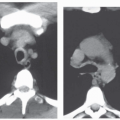
Focal Ground-Glass Opacities, Airspace Consolidation, or Both
Unilateral or bilateral patchy areas of airspace consolidation containing air bronchogram are a nonspecific radiologic pattern. In the clinical context of small airway disease, they are suggestive of BOOP, reflecting the filling of the distal airspaces with granulation tissue. Consolidation affects mainly the peribronchial or subpleural lung regions (
Fig. 5-12) (
42,
43). Small centrilobular nodular opacities may be associated, reflecting the presence of intrabronchiolar granulation tissue or peribronchiolar consolidation. In case of infectious bronchiolitis, focal areas of consolidation, reflecting areas of bronchopneumonia, can be seen in association with small centrilobular and linear opacities (
Fig. 5-8) (
15,
44).
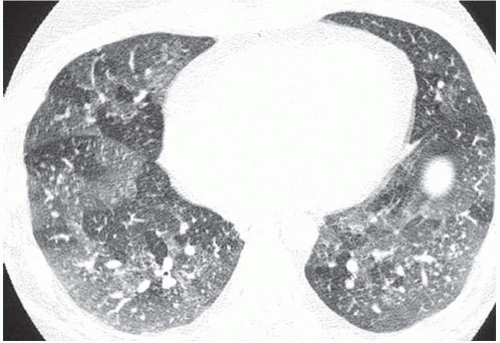
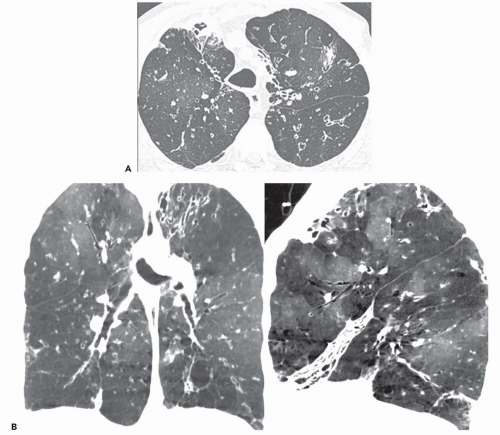
Ground-glass opacity can be seen in association with respiratory bronchiolitis (
15,
45,
46), respiratory bronchiolitis associated with interstitial lung disease (
Fig. 5-13) (
15,
47,
48), and BOOP (
42,
43). In respiratory bronchiolitis, the abnormality is bilateral, may be diffuse or patchy, and tends to involve predominantly or exclusively the upper lung zones. Ground-glass opacity is also often seen in association with airspace consolidation in BOOP. In immunocompromised patients who have BOOP, ground-glass opacity is occasionally the only abnormality on thin-section CT scans (
42). Extensive bilateral areas of ground-glass
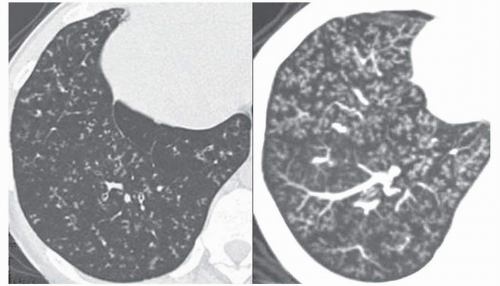
attenuation, more or less associated with poorly defined centrilobular nodular opacities, is a CT pattern frequently observed in acute or subacute hypersensitivity pneumonitis (
Fig. 5-14) (
49).
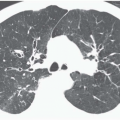
Decreased Lung Attenuation and Mosaic Perfusion
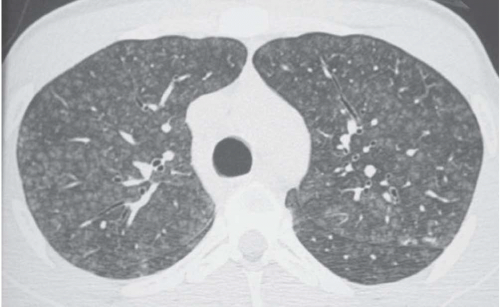
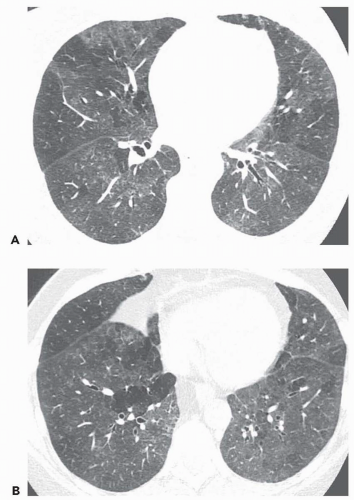
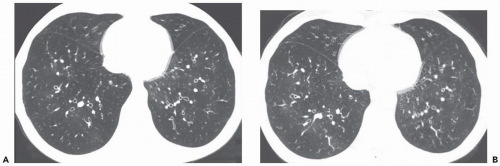
Areas of decreased lung attenuation associated with vessels of decreased caliber observed in bronchiolar disease reflect bronchiolar obstruction resulting in a decrease of perfusion (
50). In acute bronchiolar obstruction, this decrease of perfusion represents a physiologic reflex of hypoxic vasoconstriction (
51), but in the chronic state vascular remodeling takes place and the reduced caliber becomes irreversible. Although the vessels within areas of decreased attenuation on thin-section CT may be of markedly reduced caliber, they are not distorted as in emphysema. The lung areas of decreased attenuation related to decreased perfusion can be patchy or widespread. They are poorly defined or sharply demarcated, giving a geographical outline, representing a collection of affected secondary pulmonary lobules. Redistribution of blood flow to the normally ventilated areas causes increased attenuation of lung parenchyma in these areas. The patchwork of abnormal areas of low attenuation and normal lung or less diseased areas, appearing normal in attenuation or hyperattenuated, gives the appearance of mosaic attenuation (
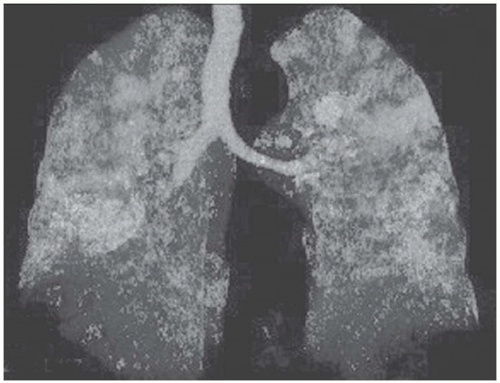
Fig. 5-15). The vessels in the abnormal hypoattenuated areas are reduced in caliber, whereas the vessels in normal areas are increased in size, and the resulting pattern is called “mosaic perfusion.” The difference in vessel size between low- and high-attenuation areas allows one to distinguish the mosaic perfusion pattern from mosaic attenuation due to an infiltrative lung disease with patchy distribution, in which the vessels have the same caliber in both high-attenuation and normal-attenuation areas (
52,
53). However, the decreased vessel size may be subtle and difficult to observe in some patients with mosaic perfusion (
Fig. 5-16) (
54).
Mosaic perfusion pattern is not always the result of bronchiolar disease and can also be caused by direct vascular obstruction (
52). The obstructed pulmonary arteries are responsible for the low-attenuation areas, whereas redistribution of blood to surrounding normal lung areas results in increased attenuation. Vascular diseases leading to mosaic perfusion pattern seen on CT images include mainly chronic thromboembolic disease
(
55,
56) and, less often, primary pulmonary hypertension (
57), pulmonary capillary hemangiomatosis (
58), pulmonary venoocclusive disease (
59), polyarteritis nodosa (
52), scleroderma (
57), and intimal sarcoma of the pulmonary arteries (
60). The differential diagnosis between a bronchiolar and a vascular cause can be based on the presence or absence of air trapping on expiratory CT, respectively (
54,
61). When caused by bronchiolar obstruction, mosaic perfusion is accentuated on expiratory CT because the low-attenuation areas show air trapping (
Fig. 5-17). In chronic vascular occlusive disease, air trapping theoretically does not occur. However, in a study by Worthy et al. (
52) applying the previously mentioned criteria to distinguish diseases that may cause mosaic pattern of lung attenuation on CT scans, two observers made a correct diagnosis in 90% of infiltrative lung disease cases, 91% of airway disease, and only 32% of vascular disease. The difficulty in the diagnosis of vascular disease was the result of the presence of expiratory air trapping in a certain number of cases (
52). This phenomenon was
confirmed recently by Arakawa et al. (
62), who found expiratory air trapping in six of nine patients with chronic embolism. Air trapping was associated with the presence of proximal arterial stenosis (
p <.01), and the area showed less contrast enhancement than did the adjacent lung (
p <.05).
Air trapping at expiratory CT is defined as “retention of excess gas (air) in all or part of the lung, especially during expiration, either as a result of complete or partial airway obstruction or as a result of local abnormalities in pulmonary compliance,” according to the Nomenclature Committee of the Fleischner Society (
63). The air is trapped and the cross-sectional area of the affected parts of the lung does not decrease in size on expiratory CT. Usually the regional inhomogeneity of the lung density seen at end-inspiration on thin-section CT scans is accentuated on sections obtained at end, or during, expiration because the high-attenuation areas increase in density and the low-attenuation areas remain unchanged (
Fig. 5-17). In the case of more global involvement of the small airways, the lack of regional homogeneity of the lung attenuation is difficult to perceive on inspiratory scans, and as a result, mosaic perfusion becomes visible only on expiratory scans. In patients with particularly severe and widespread involvement of the small airways, the patchy distribution of hypoattenuation and mosaic pattern is lost. Inspiratory scans appear with an apparent uniformity of decreased attenuation in the lungs, and scans taken at endexpiration may appear unremarkable. In these patients, the most striking features are paucity of pulmonary vessels and lack of change of the cross-sectional areas of the lung at comparable levels on inspiratory and expiratory scans (
Fig. 5-18).
Mosaic perfusion pattern associated with expiratory air trapping is seen on thin-section CT scans in patients who have obliterative bronchiolitis, regardless of etiology (
15,
64,
65); bronchiolitis associated with hypersensitivity pneumonitis (
49,
66); and asthma (
67,
68).
Lobular Areas of Expiratory Air Trapping
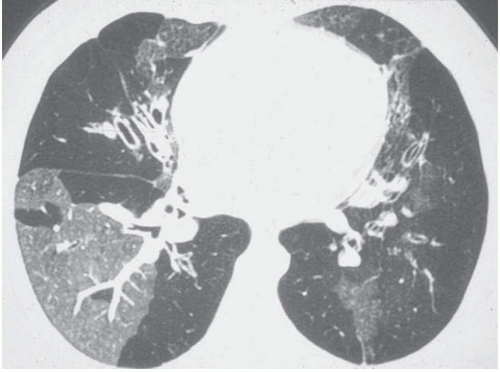
Focal areas of low attenuation may appear on expiratory CT scans, whereas no lung attenuation abnormality is depicted on inspiratory CT scans (
Fig. 5-19). This
phenomenon reflects the presence of air trapping in areas where partial airway obstruction is present (
69,
70,
71,
72). These areas are commonly well demarcated, reflecting the geometry of individual or joined lobules. This pattern is frequently observed in smokers and in patients with asthma (
Fig. 5-20), obliterative bronchiolitis, bronchiolitis associated with hypersensitivity pneumonitis (
Fig. 5-21), or sarcoidosis (
Fig. 5-19). In addition, this pattern may also be seen in patients with acute pulmonary embolism (
73). Arakawa et al. (
73) found in a series of 41 patients with acute pulmonary embolism one or more areas of air trapping in 72% of the patients. This air trapping was seen not only in areas with pulmonary embolism but also in areas without embolism. The proposed mechanism of bronchoconstriction in acute pulmonary embolism includes bronchoactive amines released from platelet aggregations in the thrombus or a change in parasympathetic nervous system tension, which centralizes the bronchial smooth muscle tension.
The diagnostic value of the pattern of lobular areas of air trapping on expiratory CT scans depends on the extent and location of air-trapping areas. In approximately 50% of asymptomatic subjects, lobular areas of air trapping may be depicted on expiratory CT scans in dependent portions of the lungs irrespective of the patient’s position (
70,
72,
74,
75,
76). Lee et al. (
74) showed that the frequency of air trapping observed on expiratory CT scans of asymptomatic subjects increases with age (
p < .05), and the degree of air trapping has a significant correlation with age (
r = 0.523,
p <.001).
The physiologic presence of focal areas of low attenuation on expiratory CT must be taken into account in the interpretation of air trapping (
64,
75). Usually, dependent lung regions show a greater increase in lung density during expiration than do nondependent lung regions. As a result, the anteroposterior attenuation gradients normally seen on inspiratory scans are significantly greater on expiratory scans. The anteroposterior lung attenuation
gradient can have a lobar component on expiratory scan; the posterior aspect of the upper lobe, anterior to the major fissure, often appears denser than the anterior aspect of the lower lobe, behind the major fissure. Some focal areas of low attenuation may also be seen near the tip of the lingula. All these physiologic low-attenuation areas involve less than 25% of the cross-sectional area of one lung at one scan level. As a result, air trapping can be considered abnormal when it affects nondependent areas or dependent lung areas greater than 25% of the lung cross-sectional area or a lung volume equal to or greater than a pulmonary segment and is not limited to the superior segment of the lower lobe.
The combination of ground-glass attenuation areas, mosaic perfusion pattern, and lobular areas of expiratory air trapping in a given patient expresses the association of interstitial and airway disease and has been called “head cheese pattern” (
40). This pattern is suggestive of hypersensitivity pneumonitis, respiratory bronchiolitis-associated intersitial lung disease (RB-ILD), and sarcoidosis.


 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access