Trachea and Central Bronchi
Tracheal disease has traditionally been difficult to identify. It has been described as the radiologic blind spot in the chest (1). Recognition of tracheal disease is notoriously difficult, especially early in its course; it has been estimated that the tracheal lumen must be occluded by more than 75% before symptoms of obstruction develop, and even then patients are frequently mistaken as being asthmatic. In a minority of cases hemoptysis may lead to early diagnosis. Although a relatively rare cause of primary disease, earlier diagnosis is now often possible with the advent of routine computed tomography (CT) imaging.
For descriptive purposes this chapter first addresses focal and then diffuse tracheal abnormalities, with the recognition that this distinction may be arbitrary. A description of tracheal anatomy and normal tracheal variants is presented in Chapter 1.
FOCAL TRACHEAL DISEASE
Tracheal Trauma
Patients suffering tracheal trauma frequently fail to live long enough to be evaluated (2). In patients who do receive medical attention, blunt trauma may also result in pulmonary contusion and/or laceration, thoracic spine injuries, esophageal disruption, or multiple fractures. Extrathoracic organ injury is also frequently encountered (3). Not surprisingly, most reported series are small. Trauma may be either penetrating or, more often, blunt (Figs. 3-1, 3-2, 3-3 and 3-4). Although penetrating tracheal injuries typically involve the neck, blunt traumatic injuries usually involve the intrathoracic portions of the trachea and often extend to the mainstem bronchi (4). Most often the result of deceleration or so-called steering wheel injuries, proposed mechanisms of injury include compression of the airways between the sternum and thoracic spine with lateral expansion of the thorax; shearing at fixation points, especially at the carina; and elevated intratracheal pressure, especially against a closed glottis (5). These injuries result in transverse lacerations; disruption of the posterior, membranous portion of the trachea; or, less commonly, shearing of the mainstem bronchi from the carina (Figs. 3-2 and 3-3). Although the entire length of the trachea is vulnerable to injury following blunt trauma more than 80% of cases occur within 2.5 cm of the carina (2). It has been suggested that the left mainstem bronchus is less frequently disrupted, probably because of its longer course through the mediastinum, which offers some extra measure of protection. Additional causes of tracheal rupture include overdistension of endotracheal tubes (Fig. 3-4) and traumatic intubation (6).
Radiographic findings most often include pneumomediastinum and deep cervical emphysema (7). Unfortunately, neither of these signs is specific for tracheal disruption. Pneumomediastinum, for example, most commonly occurs secondary to air leaks originating in the pulmonary interstitium, the so-called Macklin effect (8). Pneumothoraces are also common, presumably the result of disruption of the mediastinal pleura. In those patients in whom the airway is partly occluded by blood clots or compression by an adjacent mediastinal hematoma, the first sign of tracheal rupture may be a persistent air leak following chest tube insertion. In more severe cases, bronchial avulsion may lead to a falling away of the involved lung from the hilum resulting from disruption of central attachments, the so-called fallen lung sign (7).
CT findings in patients with blunt trauma have been reported (8, 9, 10, 11, 12). In a recent retrospective study of 14 patients with tracheal rupture either resulting from trauma or following intubation, CT proved diagnostic in 85%, providing direct visualization of tracheal tears in 10 patients (71%). Direct signs of tracheal rupture included focal wall defects in more than 50% of patients (Figs. 3-2 and 3-3); less commonly, rupture resulted in a deformed tracheal lumen. In this same series, overdistension of the tube balloon was noted to be particularly common, occurring in five of seven intubated patients. Of particular value was the finding of herniation of the endotracheal tube balloon
outside the confines of the tracheal wall (Fig. 3-4). It should be emphasized that optimal evaluation of tracheal injuries should include use of advanced image processing, especially multiplanar reconstructions. In select cases, visualization of tracheal tears may be most easily identified with virtual bronchoscopy (Fig. 3-3).
outside the confines of the tracheal wall (Fig. 3-4). It should be emphasized that optimal evaluation of tracheal injuries should include use of advanced image processing, especially multiplanar reconstructions. In select cases, visualization of tracheal tears may be most easily identified with virtual bronchoscopy (Fig. 3-3).
Although imaging findings are often suggestive, definitive diagnosis and therapy require bronchoscopy in nearly all patients. Rare exceptions include patients in whom the diagnosis can be made presumptively because of direct observation or evidence of a massive air leak following chest tube placement (13). Although treatment typically involves surgical repair, conservative therapy has been advocated for individuals with postintubation membranous tracheal ruptures if these are less than 2 cm long (6,14). Late manifestations of tracheal rupture include strictures, and patients presenting with wheezing that can be potentially misdiagnosed as asthma.
Tracheal Stenosis
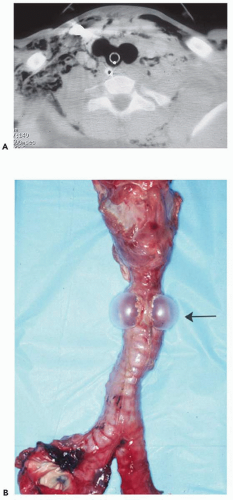
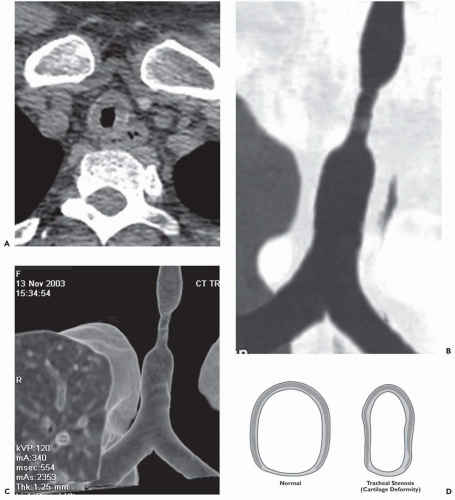
Benign airway strictures may be congenital or acquired. The most common cause of acquired strictures is iatrogenic injury resulting from intubation. Although previous reports have documented complications occurring in up to 10% to 15% of patients following intubation, their incidence has decreased dramatically since the introduction of high-volume/low-pressure balloons (15). In distinction, complications following tracheostomy tube insertion remain common, occurring in approximately 40% of patients. The two principal sites of stenosis following intubation or tracheostomy are at the stoma and at the level of the endotracheal or tracheostomy tube balloon (16). In both cases, strictures result from pressure necrosis, causing ischemia and subsequent scarring. Stenosis also may be the result of inflammation, with subsequent thinning and weakening of the tracheal wall (tracheomalacia) in the segment between the stoma and cuff; narrowing may also be caused by granulation tissue resulting from direct tracheal injury by the endotracheal or tracheostomy tube tip (16). Another complication is tracheal rupture. Rarely, tracheobrachiocephalic artery or tracheoesophageal fistulas may occur, the latter usually developing secondary to combined tracheal and esophageal intubation. Additional causes of tracheal stenosis include strictures resulting from infection, most often tuberculous, and following lung transplantation. After lung transplantation, complications typically occur at the level of the bronchial anastomosis and include dehiscence, necrosis, and stenosis from malacia, fibrous stricture, prominent granulation tissue formation, or a combination of these conditions (17, 18, 19, 20, 21).
Regardless of etiology, both CT and magnetic resonance (MR) have proved reliable methods for assessing tracheobronchial strictures (Figs. 3-5 and 3-6). In all cases, accurate evaluation requires that the precise length and degree of stenosis be assessed along with associated peritracheal or bronchial abnormalities. CT with multiplanar reconstructions, in particular, has proved of value with sensitivities of greater than 90% reported (22). It should be noted that in select cases additional certainty in assessing the degree of stenoses may also be attained by use of virtual bronchoscopy (23,24).
Although endoscopic management of tracheal stenoses has been proposed, especially with the use of lasers or stents, these typically provide only temporary benefits. Primary tracheal and laryngotracheal resection and reconstruction remain the optimal methods for treating nonneoplastic stenoses; resection up to 4 cm is feasible, which is the equivalent of eight cartilaginous rings (25). In one series of 58 patients with postintubation or idiopathic stenoses, major complications occurred in 12%, including anastomotic dehiscence and vocal cord paralysis (25).
Primary Tracheal Neoplasia
Primary tumors of the trachea are rare, representing less than 1% of bronchial neoplasms (26, 27, 28, 29) To date, a bewildering array of different tracheal tumors, both benign and malignant, have been reported, including both primary epithelial and mesenchymal neoplasms as well as secondary neoplasia resulting from either metastases or, more commonly, direct tracheal invasion by adjacent mediastinal neoplasms (30). In fact, only a few lesions occur with any frequency, and squamous cell carcinoma (SCC) and adenoid cystic carcinoma (ACC) together account for up to 86% of cases (26, 27, 28, 29). Other relatively less common malignant lesions encountered include carcinoid and mucoepidermoid tumors, while endobronchial hamartomas and bronchial papillomas are the most common benign lesions. Although these tumors are discussed separately, it should be emphasized that as a group, regardless of their cell type, most patients with endotracheal and proximal bronchial neoplasms present with a limited number of symptoms and signs: cough, wheezing, stridor, hemoptysis, and evidence of either parenchymal consolidation or atelectasis, depending on the site and degree of bronchial obstruction.
Squamous Cell Carcinoma
Squamous cell carcinoma (SCC) almost always occurs in middle-aged male smokers, paralleling the development of
these tumors in the lung. SCC has been found in association with either a previous history or concurrent or subsequent occurrence of SCC somewhere else in the respiratory tract in up to 40% of patients (27). Spread to adjacent mediastinal lymph nodes is common and carries a worse prognosis. Less often there is extension of tumor into the mainstem bronchi or adjacent esophagus, resulting in malignant esophageal-bronchial fistulas (30). On CT these lesions preferentially involve the trachea and mainstem bronchi (Fig. 3-7). Characteristically they appear as polypoid intraluminal masses; less commonly these tumors appear with eccentric, markedly irregular wall thickening and resultant luminal narrowing. Regardless of their intraluminal appearance, extension into the adjacent mediastinum or subcarinal space is common and easily identified by CT (31). In some patients SCCs may result in tracheoesophageal or bronchoesophageal fistulization. In these patients, determining the site of origin of the tumor may be problematic (Fig. 3-8). Rarely, SCCs may cause diffuse bilateral infiltration of the tracheobronchial tree. In
these patients, prebronchoscopic CT diagnosis requires a high level of clinical suspicion (Fig. 2-7).
these tumors in the lung. SCC has been found in association with either a previous history or concurrent or subsequent occurrence of SCC somewhere else in the respiratory tract in up to 40% of patients (27). Spread to adjacent mediastinal lymph nodes is common and carries a worse prognosis. Less often there is extension of tumor into the mainstem bronchi or adjacent esophagus, resulting in malignant esophageal-bronchial fistulas (30). On CT these lesions preferentially involve the trachea and mainstem bronchi (Fig. 3-7). Characteristically they appear as polypoid intraluminal masses; less commonly these tumors appear with eccentric, markedly irregular wall thickening and resultant luminal narrowing. Regardless of their intraluminal appearance, extension into the adjacent mediastinum or subcarinal space is common and easily identified by CT (31). In some patients SCCs may result in tracheoesophageal or bronchoesophageal fistulization. In these patients, determining the site of origin of the tumor may be problematic (Fig. 3-8). Rarely, SCCs may cause diffuse bilateral infiltration of the tracheobronchial tree. In
these patients, prebronchoscopic CT diagnosis requires a high level of clinical suspicion (Fig. 2-7).
Sialadenoid Tumors
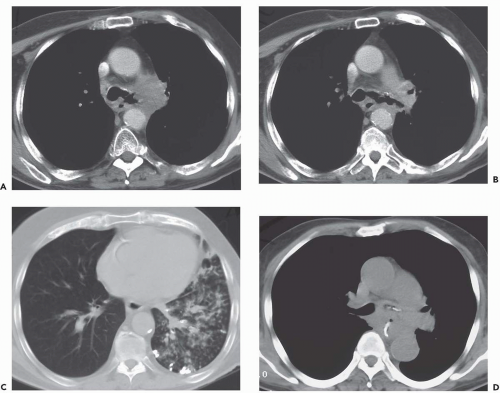
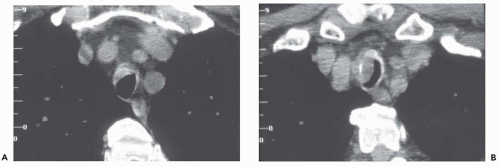
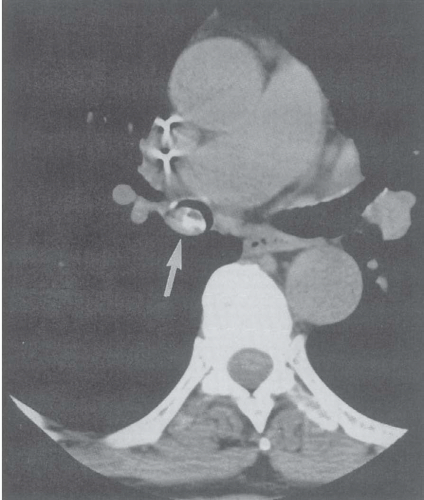
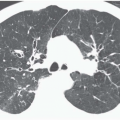
Pulmonary analogs of the salivary glands, tumors arising from tracheobronchial mucous glands primarily include cystic ACCs; less often encountered are mucoepidermoid carcinomas, which make up approximately 5% of these tumors. Other rare salivary gland tumors include mucous gland adenomas, pleomorphic adenomas, and acinic cell carcinomas (32); still more rarely reported are pulmonary oncocytomas and myoepitheliomas (33). ACCs represent low-grade malignancies that show a marked propensity for local invasion, especially invasion of submucosal and perineural lymphatics (34). Compared with SCCs, ACCs occur in slightly younger individuals with a mean age of between 40 and 50 years and show no apparent sex predilection. Furthermore, their occurrence is unrelated to cigarette smoking (27). Although lymph node metastases are relatively uncommon, especially in comparison with SCCs, metachronous hematogenous metastases are common, occurring in 44% of patients in one series (34). Most of these tumors arise from the distal trachea or proximal mainstem bronchi and appear as intraluminal polypoid masses causing luminal narrowing with invasion of adjacent mediastinal structures commonly seen (Figs. 3-9 and 3-10) (33). Rarely, they may prove multifocal in distribution (Fig. 3-11).
Unlike ACCs mucoepidermoid tumors most often are identified in segmental bronchi; in one series, they occurred in segmental bronchi in 8 of 12 patients (35). They also tend to occur in younger patients, with half less than 30 years of age (33). On CT although these lesions tend to be smaller and more circumscribed, often identifiable as solitary pulmonary or endoluminal nodules, they are otherwise indistinguishable from ACCs. As with other lesions causing airway obstruction, mucoepidermoid tumors often result in parenchymal consolidation or atelectasis. They may also extend distally to present as foci of mucoid impaction.
Carcinoid Tumors
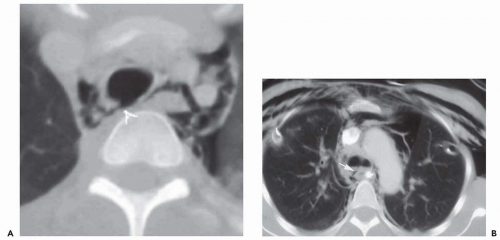
Carcinoid tumors are neuroendocrine neoplasms that arise within bronchial or bronchiolar epithelium. They are presumed to arise from existing Kulchitsky cells, neuroepithelial bodies, or pluripotential bronchial epithelial stem cells (36). They present a spectrum of histologic subtypes ranging from low- to high-grade neoplasms and are classified as typical carcinoids, atypical carcinoids, and large cell neuroendocrine carcinomas; the highest grades merge with small cell carcinoma (37). In addition to findings related to bronchial obstruction, carcinoid tumors cause hemoptysis in up to 50% of patients, reflecting their hypervascular stroma. In addition, carcinoid tumors are also known to produce a variety of hormones and neuroamines including adrenocorticotropic hormone (ACTH), serotonin, and somatostatin, among others. Cushing syndrome has been reported, although rarely. Carcinoid syndrome may also occur, although only in patients with known liver metastases (37).
Most endobronchial carcinoid tumors are centrally located. Easily identified bronchoscopically, they typically appear as smooth, cherry-red lesions with a propensity to bleed on biopsy. Although massive hemoptysis has been reported, in fact these lesions are usually safely biopsied. They tend to exhibit variable growth patterns, but most often they extend extraluminally, resulting in so-called iceberg lesions. With more peripheral lesions, airway obstruction may result in focal mucoid impaction.
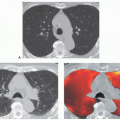
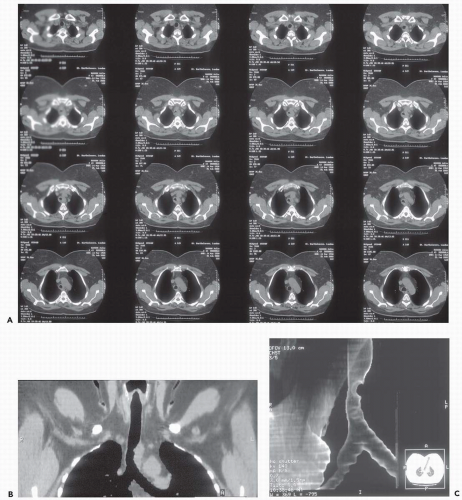 Figure 3-10 Adenoid cystic carcinoma. A: CT section through the midtrachea shows irregular soft tissue mass causing marked narrowing and slight lateral displacement of the trachea. In this case there is extensive soft tissue invasion of the adjacent mediastinal fat (compare with Fig. 3-10). Despite this finding, absence of definite encasement of adjacent mediastinal organs indicates that this tumor should still be considered resectable. B and C: Multiplanar and 3D surface rendering of this lesion, respectively, show to good advantage the true length of tracheal involvement. Note that the MPR is superior for delineating the extraluminal component of the tumor. |
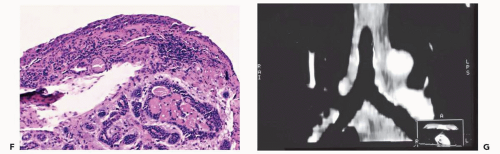
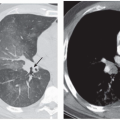
On CT, endobronchial carcinoid tumors most often present either as hilar masses or discrete intraluminal lesions (Figs. 3-12 and 3-13). A characteristic triad of features has been described, including the finding of a well-defined round or slightly lobulated tumor causing narrowing of the adjacent airway, associated with eccentric calcifications (38). These calcifications are identifiable in up to a third of patients (36,39). Carcinoid tumors are also well known to markedly enhance following administration of intravenous contrast media (Fig. 1-2; see also Fig. 3-13).
Benign Tracheal Tumors
The most common benign tracheal neoplasm is a squamous cell papilloma. Most often occurring in middle-aged male smokers, squamous papillomas typically involve the larynx or bronchi. Isolated tracheal involvement is rare and is usually limited to the tracheal wall (30). In addition to solitary neoplasms, papillomas are also classified as either inflammatory or as laryngotracheobronchial papillomatosis (40). Inflammatory polyps can be either solitary or multiple and arise in response to chronic irritation, as occurs with endobronchial foreign bodies or exposure to hot or corrosive gases. Inflammatory polyps have also been associated with broncholithiasis.
Laryngotracheal papillomatosis refers to a condition associated with multiple papillomas. Typically arising in the larynx, these lesions are histologically similar to solitary papillomas, although they most often occur in children under 5 years of age. Papillomatosis is etiologically linked to infection with human papillomavirus, either contracted at birth or acquired through sexual transmission. In this regard, papillomatosis recently has been reported to occur in patients with acquired immunodeficiency syndrome (AIDS) (Fig. 3-14). In a comprehensive literature review of 532 patients, tracheal and bronchial involvement occurred in approximately 5% of patients, whereas pulmonary parenchymal disease occurred in less than 1% (41). Unfortunately, spontaneous remission is unusual, with malignant transformation to SCC reported in adults. The origin of parenchymal disease is controversial. Because tracheobronchial and parenchymal disease is rarely encountered without coexistent laryngeal disease, it has been assumed that spread occurs by endobronchial dissemination. An association between distal spread and prior tracheostomy further supports this conclusion. Lesions typically appear first as well-defined centrilobular nodules that subsequently undergo central necrosis, resulting in multiple thin-walled cavities (Fig. 3-14). Bronchiectasis, with or without parenchymal consolidation and atelectasis, is often identified in association with nodular densities, presumably the result of bronchial occlusion and subsequent inflammation or infection.
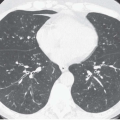
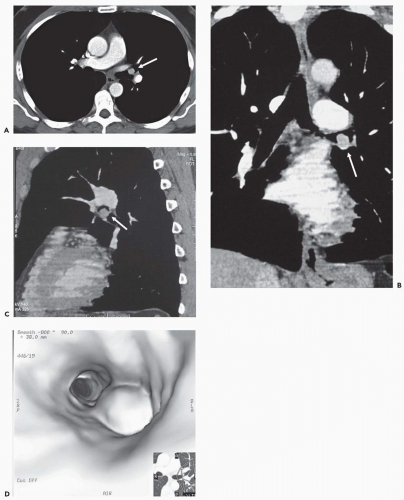 Figure 3-13 A: Axial image through the distal left upper lobe bronchus shows a well-defined, discrete intrabronchial lesion. B and C: Coronal and sagittal MPRs, respectively, and D: virtual bronchoscopic image show this same lesion to good advantage, again confirming its endobronchial location. This is the same lesion illustrated in Fig. 1-2, showing marked hypervascularity following intravenous contrast administration. |
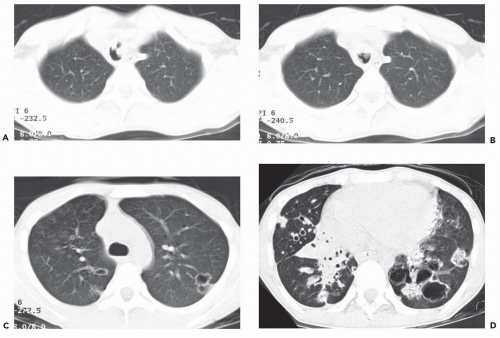 Figure 3-14 Laryngotracheal papillomatosis in a patient with AIDS. A and B: Axial CT images through the upper and midtrachea, respectively, show marked nodular irregularity of the tracheal lumen resulting from multiple polypoid lesions. C and D: Axial CT section shows typical appearance of diffuse laryngotracheal papillomatosis resulting in scattered nodules, some of which are cavitary (See also Fig. 2-20). |
Endobronchial hamartoma is another benign lesion that is occasionally encountered. Although they are the commonest benign cause of a solitary pulmonary nodule, endobronchial hamartomas are rare, estimated to represent between 10% and 20% of intrathoracic hamartomas (42). Hamartomas are mesenchymal lesions composed of a mixture of cartilage, fat, and fibrous tissue (43). They most often occur in segmental bronchi; tracheal involvement is distinctly unusual. In a report of 43 patients with documented endobronchial hamartomas, the most common presenting symptoms were recurrent respiratory tract infections and hemoptysis occurring in 44% and 33% of patients, respectively (42). On CT, although infrequently reported, the presence of fat is considered diagnostic of this entity (Fig. 3-15) (44,45).
Secondary Malignant Neoplasms
In addition to primary tumors, the trachea and central bronchi may also be secondarily involved by tumors, either as a result of direct invasion, or less commonly by hematogenous metastases (Fig. 3-16) (31). Local invasion most often results from tumors arising from the esophagus, larynx, thyroid, and lung. Metastatic disease, although less common, most often results from renal and colonic primary lesions (46). It should be emphasized that assessment
of tracheal invasion may be exceedingly difficult with CT. The finding of a peritracheal soft-tissue mass causing focal displacement of the adjacent tracheal lumen should be interpreted as nonspecific. It cannot be overemphasized that in the absence of a discrete endobronchial mass, evidence of fistulization to adjacent mediastinal structures, or destruction of tracheal cartilage, the diagnosis of tracheal or mainstem bronchial invasion is limited (Figs. 3-17 and 3-18). Definitive evaluation usually necessitates either bronchoscopic or histologic confirmation. Although it has been reported that MR may be preferable to CT in assessing invasion of the tracheal wall (47), to date routine use of MR for this purpose has not gained widespread acceptance
of tracheal invasion may be exceedingly difficult with CT. The finding of a peritracheal soft-tissue mass causing focal displacement of the adjacent tracheal lumen should be interpreted as nonspecific. It cannot be overemphasized that in the absence of a discrete endobronchial mass, evidence of fistulization to adjacent mediastinal structures, or destruction of tracheal cartilage, the diagnosis of tracheal or mainstem bronchial invasion is limited (Figs. 3-17 and 3-18). Definitive evaluation usually necessitates either bronchoscopic or histologic confirmation. Although it has been reported that MR may be preferable to CT in assessing invasion of the tracheal wall (47), to date routine use of MR for this purpose has not gained widespread acceptance
Endobronchial Neoplasms: Management and Prognosis
Given the overlap in both clinical manifestations and CT appearance of most endobronchial lesions, not surprisingly definitive diagnosis requires histologic evaluation in nearly all cases. In addition to the primary tracheal lesions mentioned previously, it should be noted that a bewildering variety of additional rare endobronchial lesions have been reported (27,48,49). Regardless of origin, primary endotracheal lesions are best treated surgically, especially with primary resection and reanastomosis, usually followed by radiation. To date, most large series have primarily focused on patients with SCCs and ACCs. In a series of 20 patients with primary tracheal neoplasms treated surgically, Hazama et al. (29) reported a 5-year survival rate of 72.3% for patients undergoing surgical treatment including segmental tracheal, laryngotracheal, or carinal resections. Nearly identical results have been reported by others (27,50). Overall, approximately three quarters of patients with primary tracheal or carinal lesions will be resectable.
In an evaluation of 198 patients with primary tracheal tumors reported by Grillo et al. (27), 147 (74%) were treated surgically, including 44 with SCCs, 60 with ACCs, and 43 with assorted benign and malignant tumors. Surgical mortality for primary resections was 5%. Patients with ACCs survived longer than those with SCCs, especially when the latter were associated with mediastinal adenopathy. Curiously, in patients with ACCs, survival was similar whether or not there was evidence of mediastinal nodes or residual tumor along the margins at resection. Nearly identical results have been reported by Hazama et al. (29), who also noted that survival in patients with ACCs was independent of the presence of positive margins at surgery. In this setting, it turns out that the benefits of performing primary resection outweigh the potential liability of leaving behind residual microscopic tumor.
Although surgical resection is to be preferred when feasible, an increasing number of nonsurgical options are now available for the treatment, and especially the palliation, of endobronchial tumors. As discussed in depth in Chapter 2, these include neodymium:yttrium-aluminum-garnet (Nd:YAG) laser phototherapy, photodynamic therapy following the intravenous administration of photosensitizing drugs, cryotherapy, electrocoagulation, brachytherapy, and endobronchial stents with or without prior bronchoplasty (Figs. 3-12 and 3-19) (51).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree