LEARNING OBJECTIVES
1. Name the three most common causes of a solitary pulmonary nodule (SPN).
2. Name four important considerations in the evaluation of an SPN.
3. Define the terms pulmonary nodule and pulmonary mass.
4. Name six common causes of cavitary nodules.
5. Name four common causes of multiple pulmonary nodules.
6. Describe the indications for percutaneous biopsy of an SPN.
7. Describe the most frequent complications related to percutaneous lung biopsy using computed tomographic (CT) or fluoroscopic guidance.
8. Describe the role of positron emission tomography in the evaluation of an SPN.
9. Describe an appropriate imaging algorithm to evaluate an SPN.
10. Describe an appropriate algorithm for management of small incidental pulmonary nodules detected on CT scans.
A pulmonary nodule is defined as “a rounded opacity, well or poorly defined, measuring up to 3 cm in diameter” (1). A pulmonary mass is distinguished from a nodule on the basis of size and is defined as “any pulmonary, pleural, or mediastinal lesion seen on chest radiographs as an opacity greater than 3 cm in diameter (without regard to contour, border, or density characteristics). Mass usually implies a solid or partly solid opacity” (1). Some authors, however, use the terms nodule and mass interchangeably (2). A nodule seen on computed tomographic (CT) scan is defined as a “small, approximately spherical, circumscribed focus of abnormal tissue” (3). Definitions are helpful but imprecise; in practice, there is variability in what is termed a pulmonary nodule or mass. The differential diagnoses are different for solitary and multiple pulmonary nodules, and thus each will be discussed separately in this chapter.
SOLITARY PULMONARY NODULES
Solitary pulmonary nodules (SPNs) are very common. A radiologist in an active practice may see one or more per day. Many more are not perceived. At chest radiography, an SPN is seldom evident until it is at least 9 mm in diameter (4), and nearly 90% of newly discovered SPNs on chest radiographs may be visible in retrospect on available prior radiographs (5). The importance of an SPN derives from the frequency with which it represents a primary lung cancer. The causes of an SPN are many (Table 7.1), but more than 95% fall into one of four groups: malignant neoplasms, either primary or metastatic; infectious granulomas, either tuberculous or fungal (Fig. 7.1); benign tumors, notably hamartoma; and normal intrapulmonary (perifissural) lymph nodes (2). In addition to specific radiologic features, clinical history is important in considering the likely cause of an SPN (Fig. 7.2). Lung cancer is rare in patients under 30 years of age and is more common in cigarette smokers than nonsmokers. History of a known primary tumor makes a metastasis more likely than a new (or second) primary tumor. Certain regions of the country are endemic for fungal disease and therefore have a higher prevalence of benign nodules than other regions.
Management options with an SPN include comparison with older exams, further imaging (usually CT or positron emission tomography [PET]/CT), declaration of benign etiology and no further evaluation, follow-up (usually with CT), or tissue diagnosis (either with percutaneous or transbronchial biopsy or via surgical resection). Management algorithms should take into account radiologic and clinical factors. Such additional information may obviate further workup (Fig. 7.3).
Two rules must be remembered when evaluating an SPN: first, a “nodule” is not confirmed to be within the lung unless it is seen in the lung on both posteroanterior (PA) and lateral chest radiographs or it is seen in the lung on a chest CT. Second, the current radiograph or CT scan should be compared with prior radiologic studies, when available, to confirm the chronicity of the nodule. Following these two basic rules will, on occasion, prevent unnecessary further workup and concern.
Four important considerations in the evaluation of an SPN are (i) attenuation characteristics, (ii) rate of growth, (iii) shape, and (iv) size. Each of these will be discussed separately; however, it should be noted that no single radiologic feature or combination of features is entirely specific for lung cancer or other primary malignant tumors. A definitive diagnosis may be possible based on the CT scan findings. For example, a nodule seen on chest radiography that is of fluid attenuation and conforms to a fissure is consistent with loculated pleural fluid, otherwise known as a “vanishing pseudotumor” (Fig. 7.4).
Table 7.1 CAUSES OF SOLITARY PULMONARY NODULES
Neoplastic: Malignant
Lung cancer
Solitary metastasis
Lymphoma
Carcinoid tumor
Neoplastic: Benign
Hamartoma
Benign connective tissue and neural tumors (e.g., lipoma, fibroma, neurofibroma)
Inflammatory
Granuloma
Lung abscess
Rheumatoid nodule
Inflammatory pseudotumor (plasma cell granuloma)
Congenital
Arteriovenous malformation
Lung cyst
Bronchial atresia with mucoid impaction
Miscellaneous
Pulmonary infarct
Intrapulmonary lymph node
Mucoid impaction
Hematoma
Amyloidosis
Normal confluence of pulmonary veins
Mimics of SPN
Nipple shadow
Cutaneous lesion (e.g., wart, mole)
Rib fracture or other bone lesion
“Vanishing pseudotumor” of congestive heart failure (loculated pleural effusion)
SPN, solitary pulmonary nodule
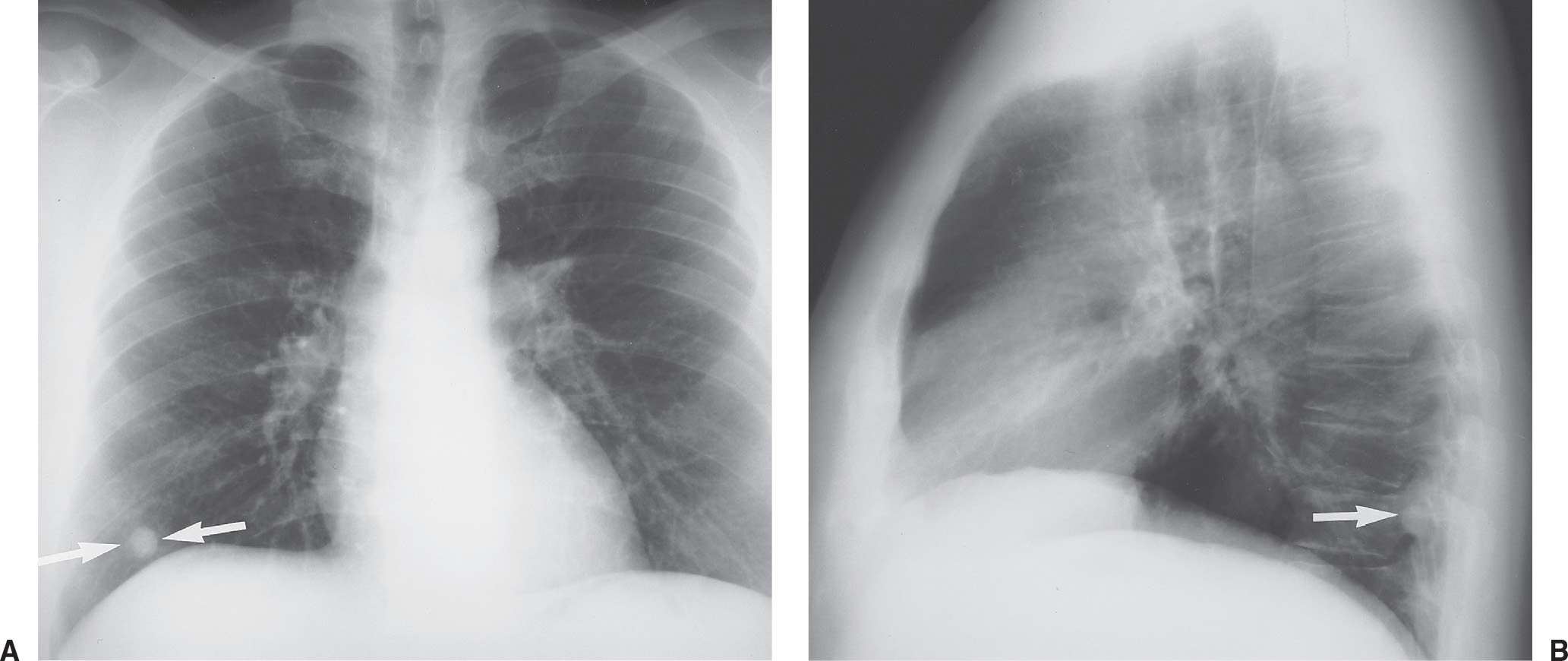
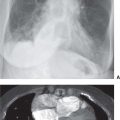
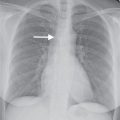
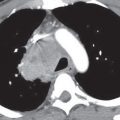
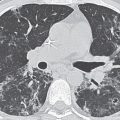
FIG. 7.1 • Granuloma. A: PA chest radiograph shows a small, well-circumscribed, round opacity at the right lung base (arrows). B: Lateral view shows that the opacity is within the lung on two views (posterior segment of the right lower lobe) and thus represents a pulmonary nodule (arrow). The high density of the nodule relative to its small size indicates that it is densely calcified. The appearance is characteristic of a benign calcified granuloma, and no further evaluation of the nodule is needed (an exception would be in a patient with a known calcium-producing primary tumor, such as osteosarcoma, which can lead to calcified pulmonary metastases; in this case, older radiographs confirmed that the nodule was stable for over 2 years).
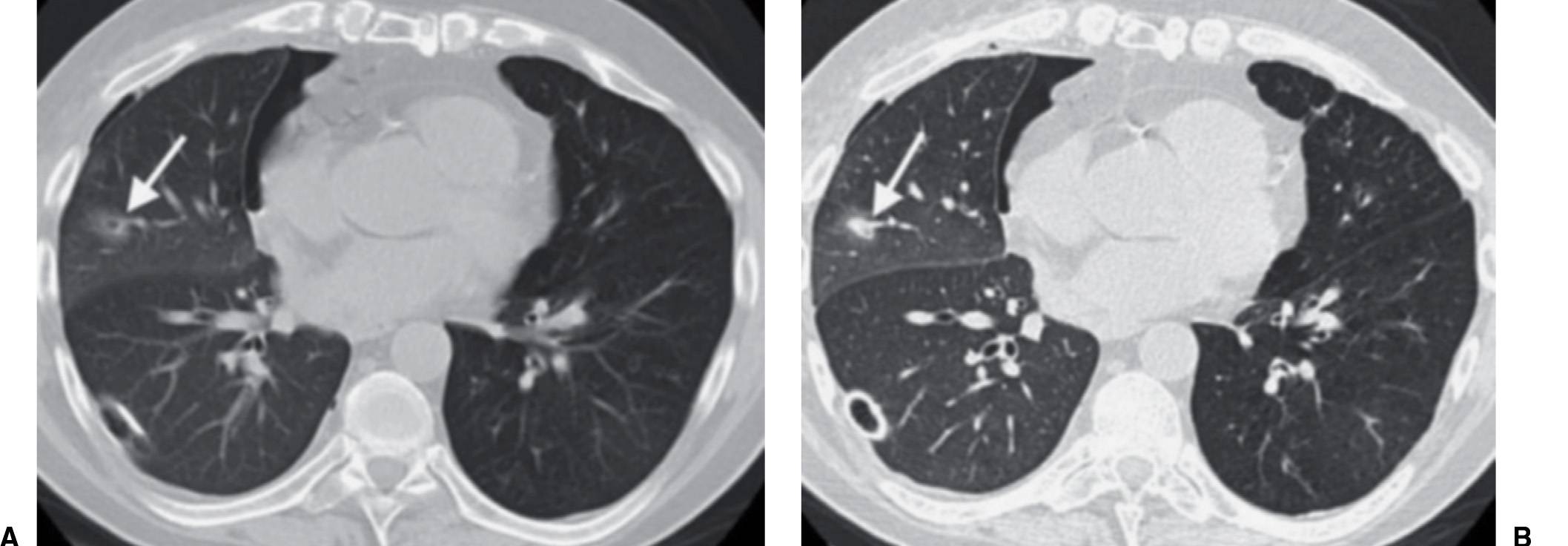
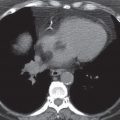
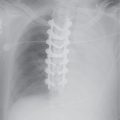
FIG. 7.2 • Postbiopsy hematoma. This 62-year-old man underwent transbronchial biopsy of the right middle lobe 11 days after lung transplantation. The history supports hematoma as the cause of the nodule in the right lung. A: CT scan shows a ground-glass nodule with central cavitation (arrow) in the right middle lobe. B: At a more inferior level, the nodule (arrow) appears more solid.
The presence or absence of calcium is the most important feature that distinguishes benign from malignant nodules. Unfortunately, 45% of benign nodules are not calcified (6). Benignity can be confirmed confidently if the lesion is smaller than 3 cm in diameter, is smoothly marginated, and exhibits one of the following patterns of calcification: large central nidus, laminated, popcorn, or diffuse. All other patterns of calcification are less specific, as further described later. SPNs smaller than 9 mm in diameter are rarely visible on chest radiographs, and a nodule of this size that is clearly seen is likely to be diffusely and densely calcified, and therefore benign (see Fig. 7.1). Dual-energy subtraction chest radiography, and some newer bone suppression software algorithms, may better depict calcification in pulmonary nodules compared with conventional chest radiography (7). The so-called popcorn calcifications, which are randomly distributed, often overlapping rings of calcium, are seen when cartilage is present, such as with a hamartoma (Fig. 7.5). Large areas of dystrophic calcium are essentially diagnostic of benign nodules, usually granulomas, and the exceptions are extremely rare. Any calcium in a nodule makes the nodule more likely to be benign. When calcium is detected on thin-section CT (≤3-mm sections), even when not diffuse and not obviously benign in nature, the likelihood of benignity is high enough to warrant follow-up imaging. This management is recommended, however, only for nodules that are otherwise smoothly marginated, 3 cm or smaller in diameter, and not increasing in size at a rate compatible with lung cancer (Fig. 7.6) (8). The presence of eccentric calcium in a nodule occasionally represents a sign of “scar carcinoma,” where the cancer has arisen from a granuloma or engulfed a fibrotic, calcified granuloma. Central calcification in a spiculated SPN should be viewed with suspicion, because most benign SPNs have smooth or minimally lobulated margins. Calcifications in lung cancers may appear amorphous, stippled, or diffuse, and some can have dense foci of calcification or be entirely calcified, with a pattern resembling that of benign disease. These last two patterns can be seen in carcinoid tumors, metastatic osteosarcoma, and chondrosarcomas. Stippled calcification can be seen in metastases from mucin-secreting tumors such as colon or ovarian cancers.
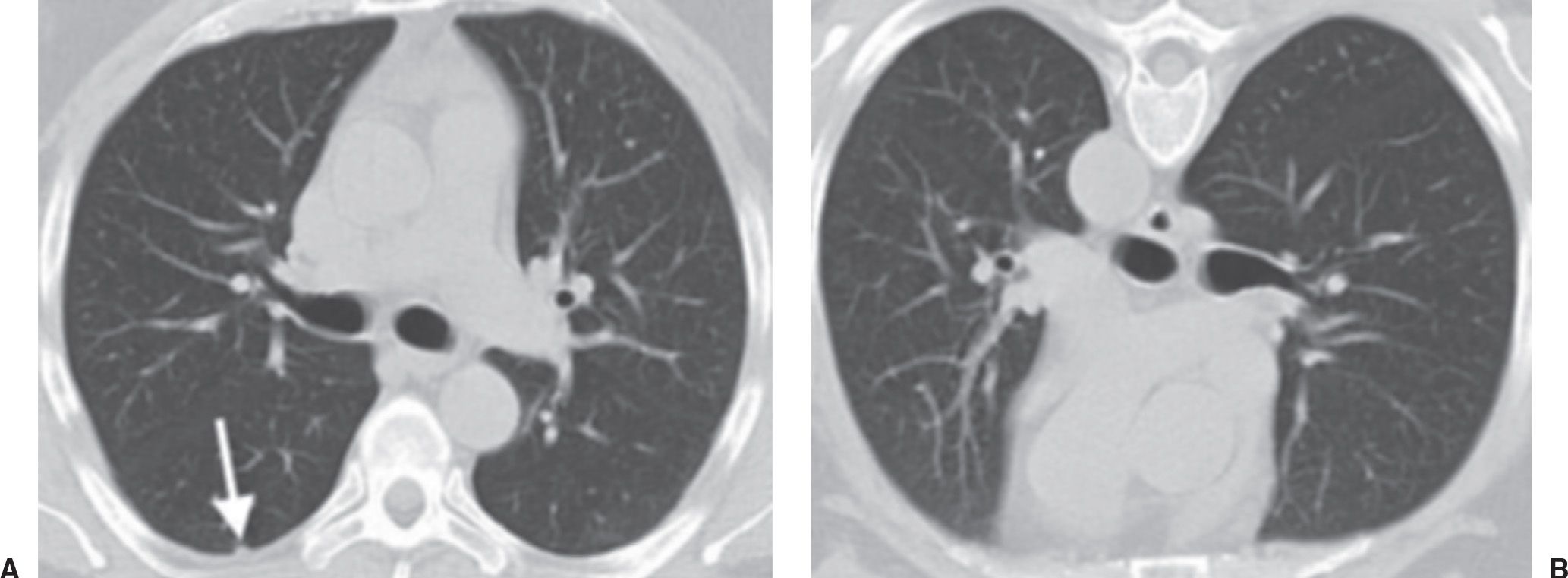
FIG. 7.3 • Dependent atelectasis. A: Supine CT image shows a small, rounded opacity (arrow) in the dependent right lower lobe. B: The “nodule” disappears on prone imaging, confirming the etiology to be focal atelectasis.
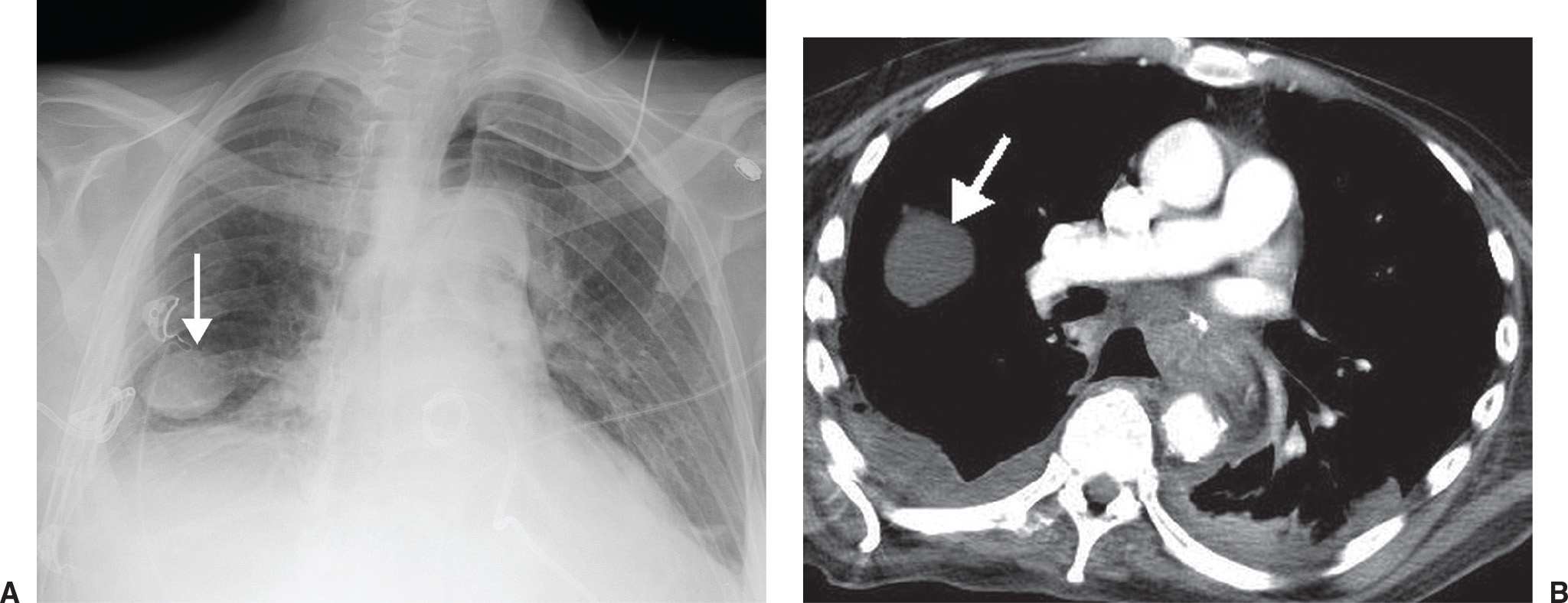
FIG. 7.4 • Pseudotumor. A: PA chest radiograph shows an ovoid, circumscribed mass in the right lower hemithorax (arrow). A chest tube projects over the right costophrenic angle. B: CT scan shows the mass (arrow) to be of fluid attenuation and in the expected location of the major fissure, consistent with a pleural fluid collection. Pleural fluid is also seen in the posterior right pleural space.
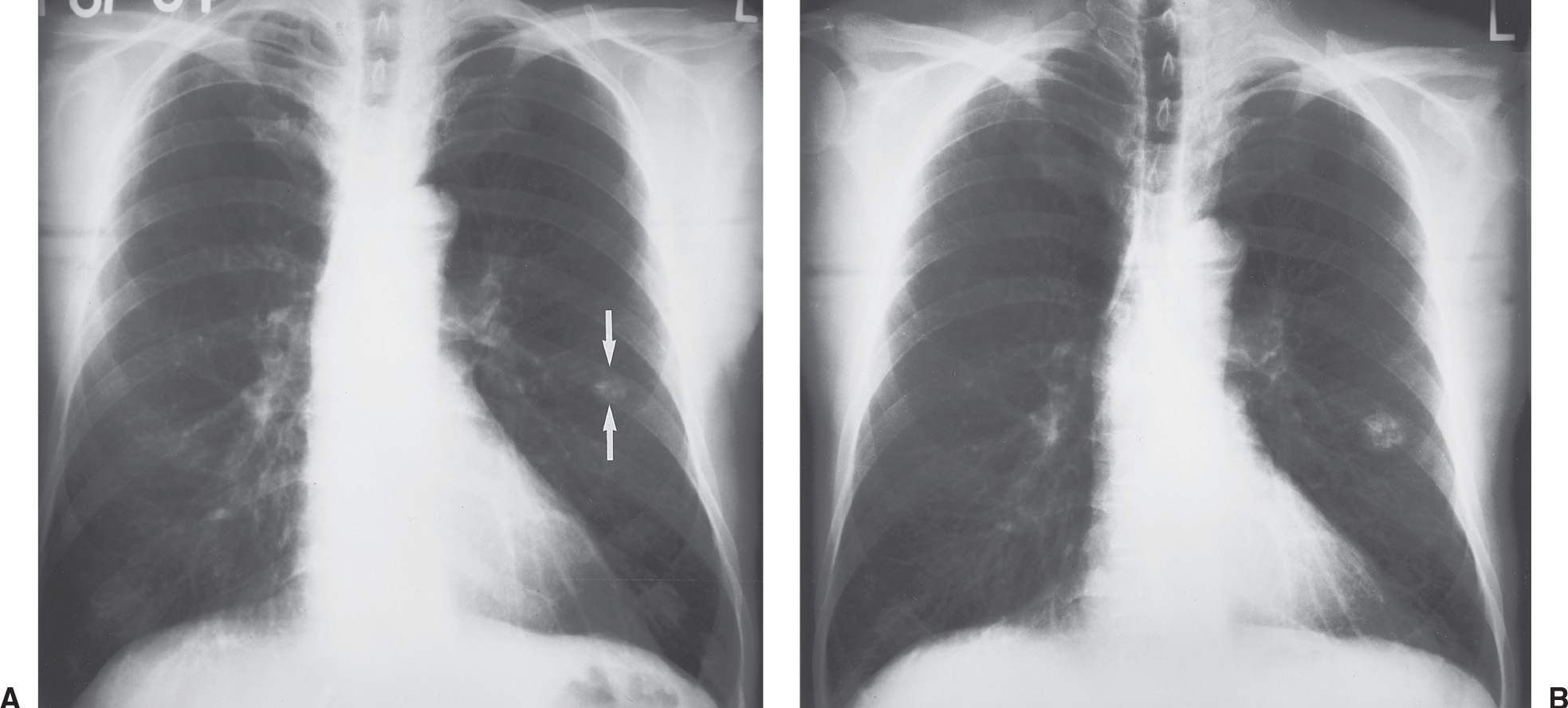
FIG. 7.5 • Hamartoma. A: PA chest radiograph shows a round, calcified opacity in the left middle lung (arrows). B: PA chest radiograph obtained 5.5 years later shows enlargement of the nodule, which has doubled in volume. The randomly distributed calcifications, arranged in overlapping rings, now have the typical “popcorn” appearance described with hamartomas. It is not unusual for hamartomas to enlarge; unlike malignant nodules, however, the growth rate is slow, with doubling occurring in more than 18 months’ time.
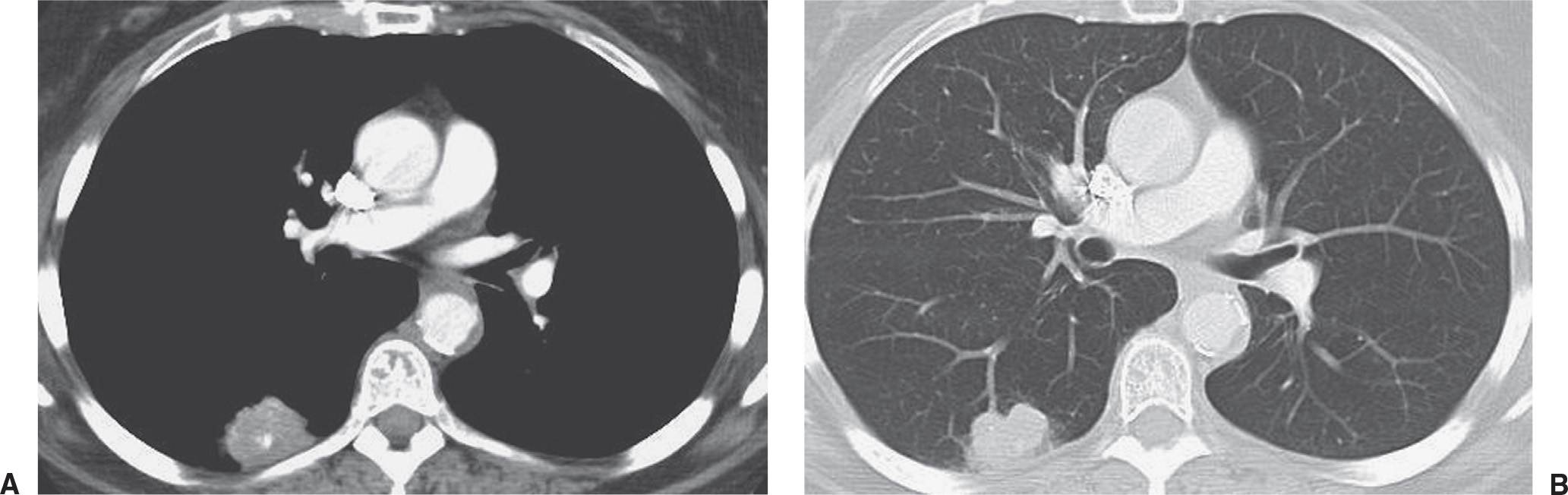
FIG. 7.6 • Small-cell carcinoma. A: CT scan shows an irregular, calcified, round nodule in the right lower lobe. The pattern of calcification is not definitely benign, and the nodule’s irregular margin makes it suspicious for neoplasm. B: CT scan with lung windowing shows the nodule to have a lobulated contour.
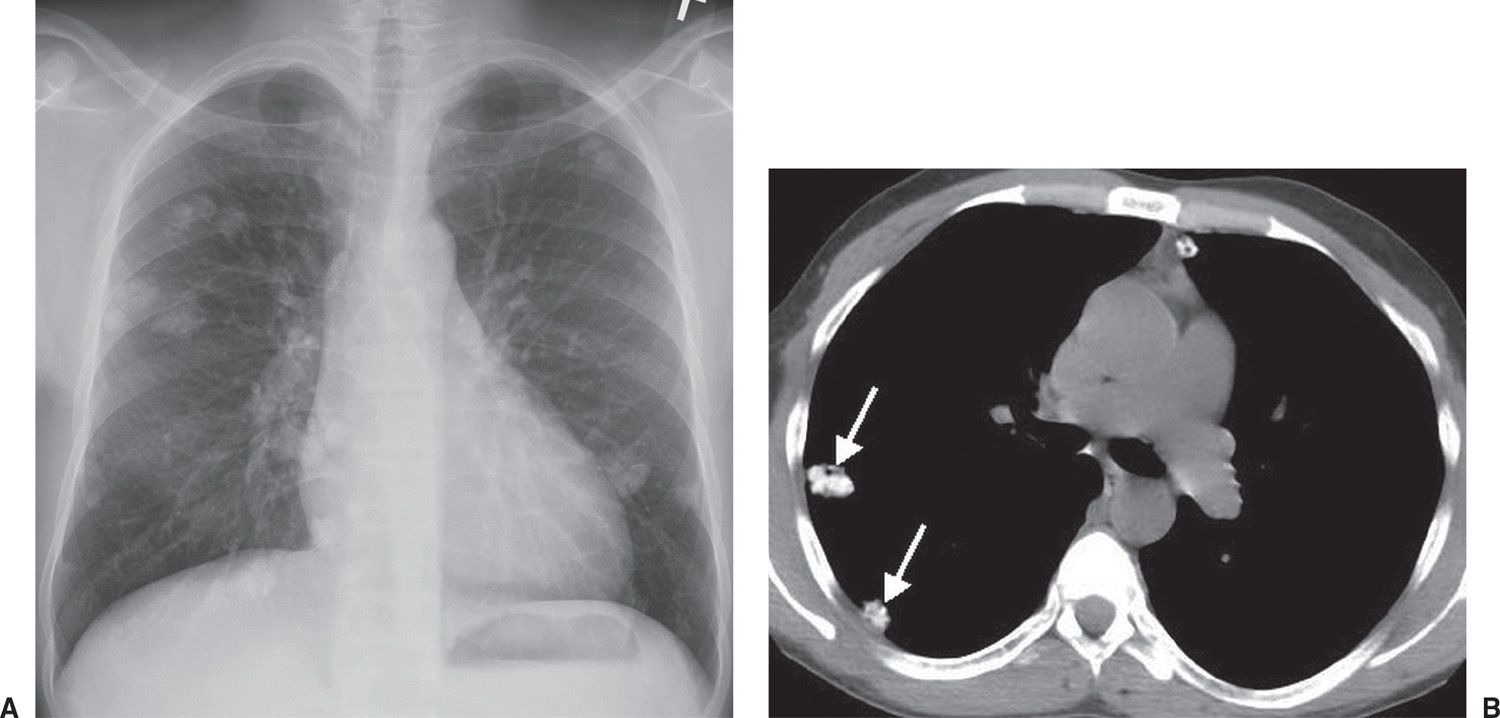
FIG. 7.7 • Metastatic calcification. A: PA chest radiograph of a 37-year-old man with renal failure and hyperparathyroidism shows multiple dense pulmonary nodules of varying size. B: CT scan confirms multiple calcified nodules (arrows).
Metastatic calcification is deposition of calcium salts in otherwise normal tissue, due to elevated serum levels of calcium in blood. This can occur with disorders of metabolism as well as increased absorption or decreased excretion of calcium and related minerals, as seen in hyperparathyroidism (9). In contrast, dystrophic calcification is caused by abnormalities or degeneration of tissues resulting in mineral deposition, though blood levels of calcium remain normal. These differences in pathology also mean that metastatic calcification is often found in many tissues, whereas dystrophic calcification is localized. Metastatic calcification can occur widely throughout the body but principally affects the interstitial tissues of the vasculature, kidneys, lungs (Fig. 7.7), and gastric mucosa. For the latter three, acid secretions or rapid changes in pH levels contribute to the formation of salts.
Pulmonary hamartomas are benign lesions consisting of an abnormal mixture of the normal constituents of the lung. If fat is present within a nodule, the most likely diagnosis is hamartoma, and less likely diagnoses are lipoma or myelolipoma. Exceptions include metastatic liposarcoma or renal cell carcinoma, which rarely present as fat-containing nodules on CT. Most pulmonary hamartomas contain masses of cartilage and may also contain fat or cystic collections of fluid (Figs. 7.8 and (7.9). They grow slowly and are usually solitary (see Fig. 7.5). More than 90% are peripheral in location (10). Pulmonary hamartomas can range up to 10 cm in diameter, although most are less than 4 cm, and are usually spherical, lobulated, or notched with a very well-defined edge. In patients without prior malignancy, focal fat attenuation (−40 to −120 Hounsfield units) is a reliable indicator of a hamartoma (11).
A recent category of nodules is described as “perifissural.” A recent study reported that perifissural nodules (PFNs) detected in asymptomatic heavy smokers participating in a screening study are almost certainly benign and do not require follow-up (12). PFNs were defined as typical (fissure-attached, homogeneous, solid nodules with smooth margins and an oval, lentiform, or triangular shape) or atypical (have all the features of a typical PFN but are not visibly attached to a fissure or fissure-attached nodules that are convex on one side and rounded on the other). Spiculated or spherical nodules are not classified as PFNs.
On CT, nodules can be described as solid, partly solid, or nonsolid. Aerated lung is visible through a nonsolid (ground-glass) nodule. Whereas most cancerous nodules are solid, partly solid nodules are most likely to be malignant and are often caused by adenocarcinoma in situ (formerly bronchioloalveolar carcinoma) (Figs. 7.10 to 7.12). Air bronchograms and bronchiolograms are seen more commonly in pulmonary carcinomas than in benign nodules.
An SPN that exhibits no growth for at least 2 years is generally considered benign (13). However, even benign lesions, such as granulomas, can grow slowly. Therefore, growth alone cannot be used to predict malignancy. Lung cancers usually take between 1 and 18 months to double in volume (14). A volume doubling is a change in diameter of about 1.25 times the previous diameter. Doubling times that are faster than 1 month suggest infection, infarction, histiocytic lymphoma, or a fast-growing metastasis from tumors such as germ cell tumor, lymphoma, melanoma, and soft tissue sarcoma (Table 7.2) (15). Doubling times slower than 18 months suggest granuloma, hamartoma, bronchial carcinoid, salivary gland adenoid cystic carcinoma, thyroid carcinoma metastases, and round atelectasis. However, there are exceptions, and cancerous tumors with doubling times of more than 730 days may appear stable during a 2-year observation period, which is why some oncologists will perform nodule surveillance for 3 or more years in certain circumstances. Growth is more accurately assessed on CT than on chest radiography, and diameters measured with electronic calipers are preferable to diameters measured manually. However, many factors, inherent and technical, including size of the nodule and determination of the nodule margin, can limit the accuracy of measurement. It is difficult to reliably show changes in size when nodules are smaller than 2 mm, and even a substantial increase in volume may be missed with small nodules.
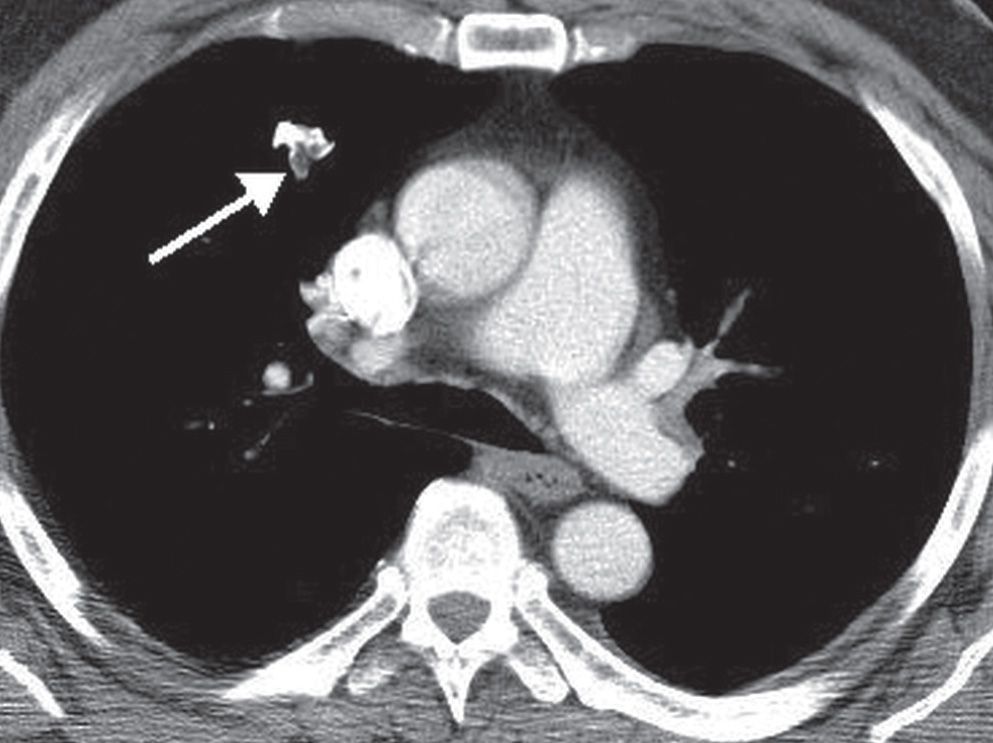
FIG. 7.8 • Hamartoma. CT scan shows an irregular right upper lobe nodule (arrow) containing high-density calcium and low-density fat.
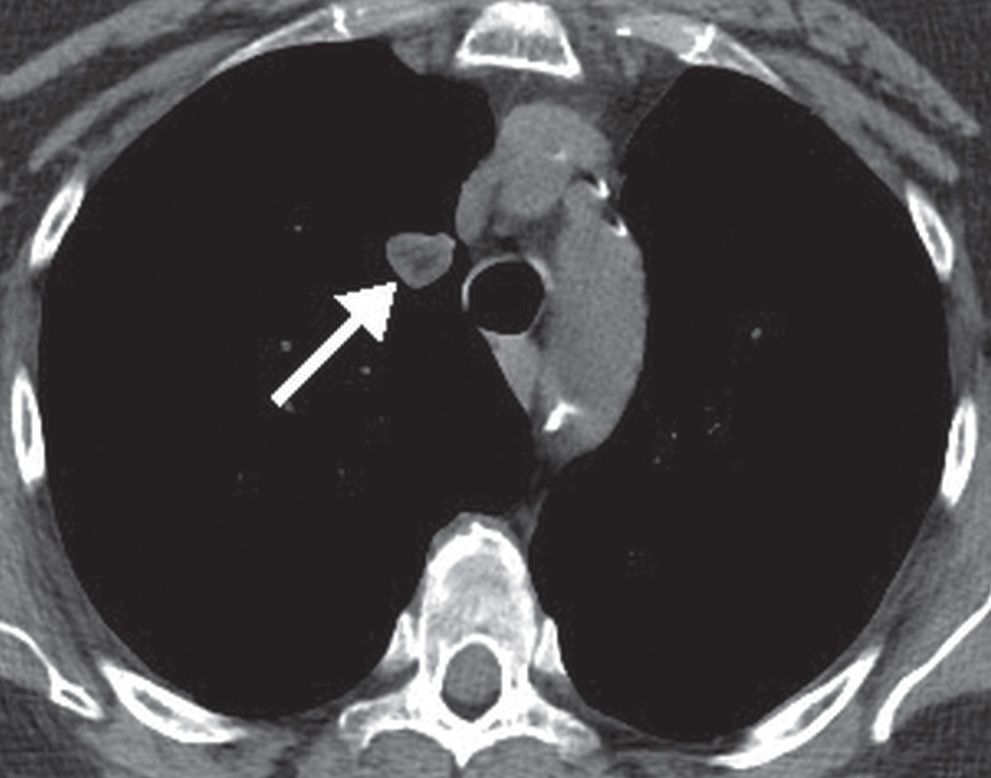
FIG. 7.9 • Hamartoma. CT scan shows a right upper lobe nodule (arrow) with central low-density fat.
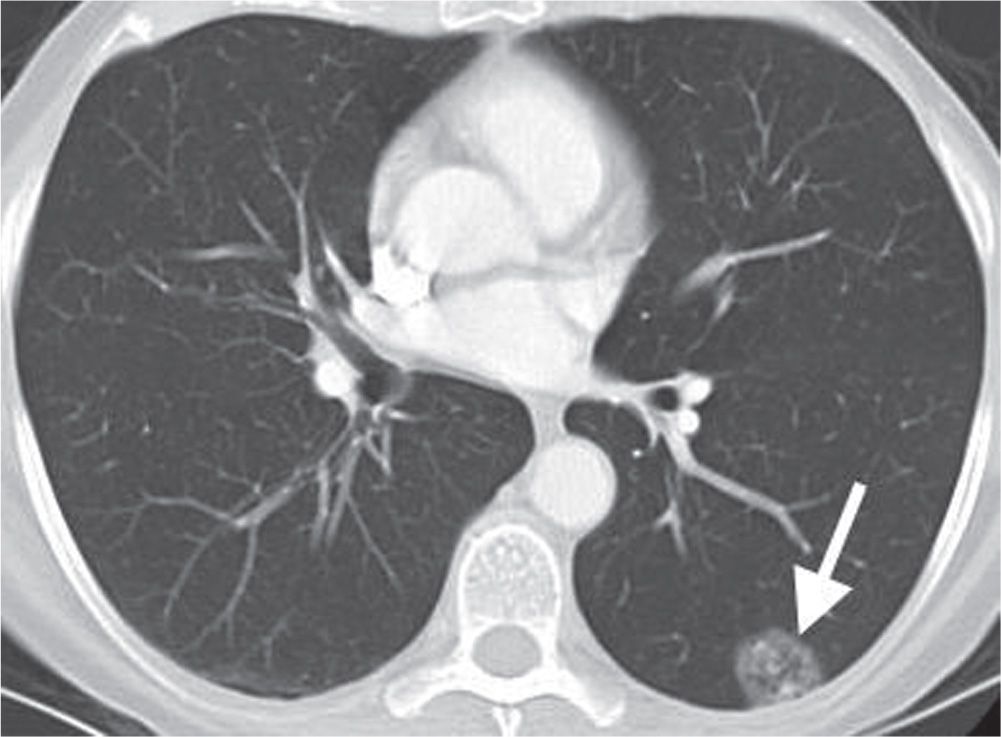
FIG. 7.10 • Adenocarcinoma in situ. CT scan shows a mixed solid and ground-glass nodule in the periphery of the left lower lobe (arrow). Internal “bubble” lucencies are a characteristic feature of this type of neoplasm.
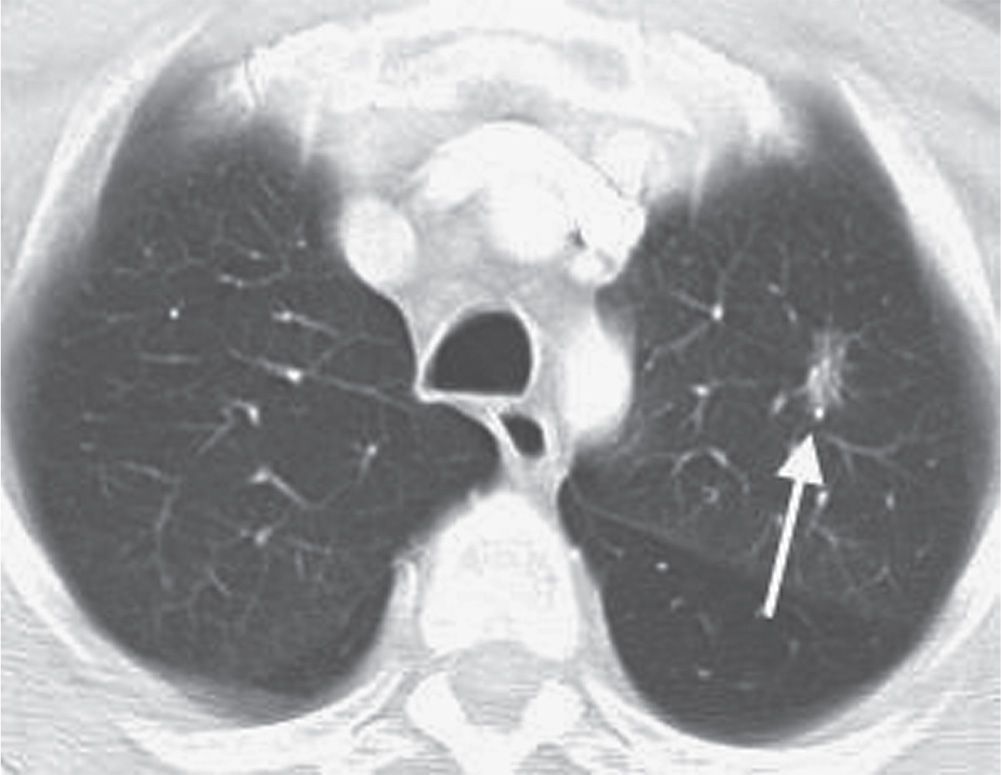
FIG. 7.11 • Adenocarcinoma in situ. CT scan shows a mixed solid and ground-glass nodule in the left upper lobe (arrow).
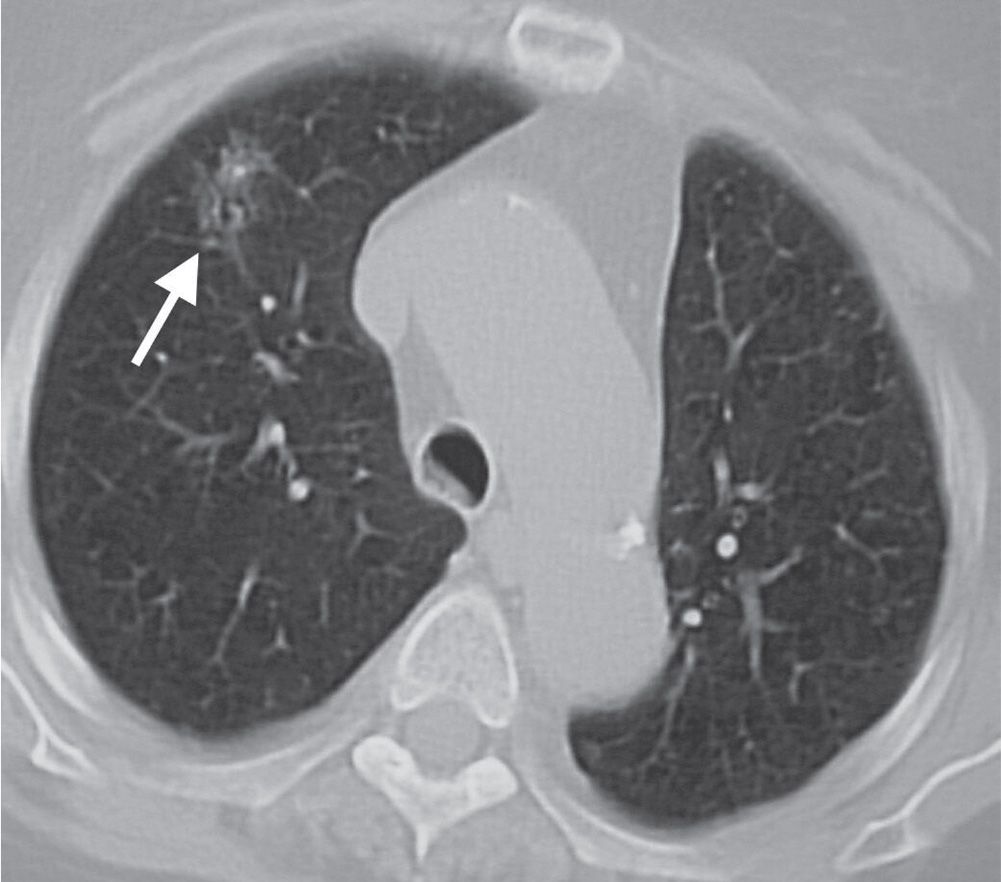
FIG. 7.12 • Adenocarcinoma in situ. CT scan shows a poorly defined ground-glass nodule in the right upper lobe (arrow).
Edge characteristics indicative of malignancy include irregularity, spiculation, and lobulation (Fig. 7.13) (16). A corona radiata, described as numerous strands radiating from the nodule into the surrounding lung, is very suggestive of lung cancer, although there are exceptions in which a corona radiata is seen in benign lesions such as infectious granulomas and other chronic inflammatory lesions (17). The “tail sign” consists of a linear opacity that extends from a peripheral nodule to the visceral pleura; for some years this sign was regarded as a reliable sign of malignancy. Studies have shown, however, that up to half of nodules showing the tail sign represent benign granulomas (18), and therefore the tail sign is a nonspecific feature of peripherally located pulmonary lesions that cannot be used to distinguish a benign from a malignant lesion. Lobulation and notching are seen with both benign and malignant nodules and are not very useful discriminating features (Figs. 7.14 and (7.15). A well-defined, smooth, nonlobulated edge is most compatible with hamartoma, granuloma, or metastasis. However, a smooth margin does not indicate benignity, as up to one-third of malignant lesions have smooth margins (Fig. 7.16) (19). Therefore, the previously mentioned signs are more helpful in their absence. Adjacent tiny nodules, called satellite nodules, are strongly associated with benignity (see Fig. 7.14) but do not allow a confident diagnosis of benignity, as 10% of dominant nodules with satellite nodules will be malignant (Fig. 7.17) (6).
Table 7.2 FAST-GROWING PULMONARY METASTASES
“Loves to Multiply Swiftly”
Lymphoma
Testicular germ cell tumor
Melanoma
Soft tissue sarcoma (osteosarcoma)
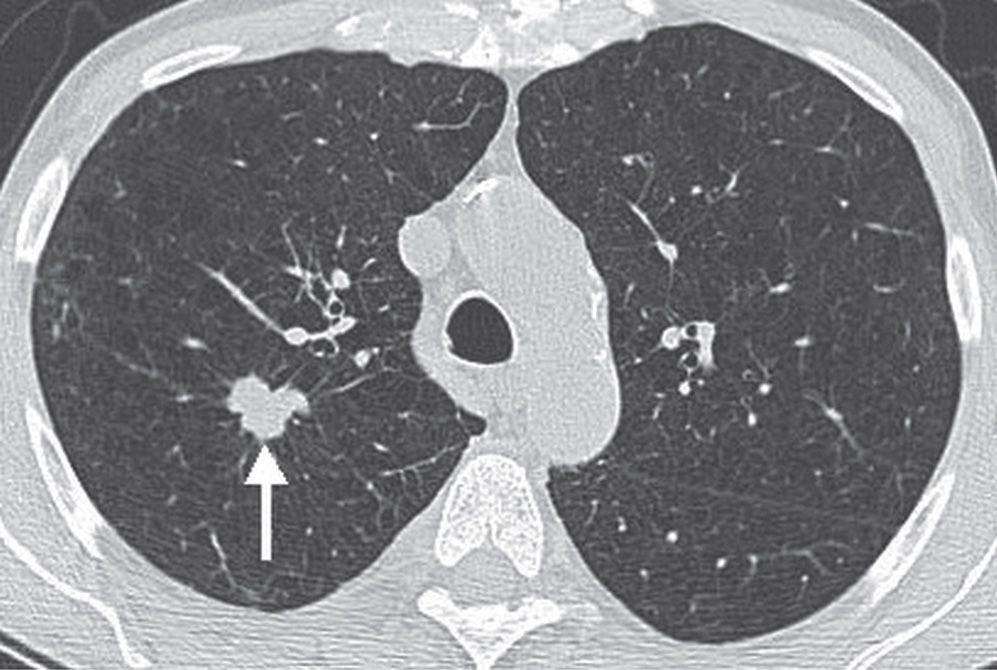
FIG. 7.13 • Bronchogenic adenocarcinoma. CT scan shows a lobulated, spiculated nodule in the right upper lobe (arrow).
SPNs with irregular-walled cavities thicker than 16 mm tend to be malignant, whereas benign cavitated lesions usually have thinner, smoother walls. However, because there is considerable overlap, cavity wall characteristics cannot be used to confidently differentiate benign and malignant SPNs (Figs. 7.18 and (7.19) (20).
Thin-section contrast-enhanced imaging of nodules at least 10 mm in diameter, with images acquired at 1-minute intervals up to 4 minutes after administration of contrast material, is a technique that has been described as a way to indicate the likelihood of benignity. Although enhancement of more than 15 Hounsfield units is more likely to represent malignancy, the false-positive rate is high and is caused by active inflammatory disease such as granulomas or organizing pneumonias. Therefore, nodule enhancement is a sensitive but nonspecific indicator of malignancy (21).
PET/CT with fluorine-18-fluorodeoxyglucose is routinely used to determine whether an SPN larger than 1 cm in diameter has malignant or benign metabolic features (22). For SPNs 1 to 3 cm in diameter, sensitivity and specificity are approximately 94% and 83%, respectively (23). False-positive PET/CT findings are associated with focal infections, inflammation, and granulomatous diseases such as tuberculosis and sarcoidosis. False-negative PET/CT findings are seen with carcinoid and adenocarcinoma in situ (formerly bronchioloalveolar carcinoma), tumors that have a low metabolic rate. Sensitivity and specificity of PET/CT decreases with nodules smaller than 1 cm in diameter.
Radiologists are frequently asked to perform percutaneous fine-needle aspiration biopsy (FNAB) of pulmonary nodules. CT allows for biopsy of many nodules as small as 5 mm in diameter. In patients who are not candidates for surgery, FNAB can be performed to confirm and determine the histologic type of malignancy. In patients who are candidates for surgery, FNAB can confirm benign disease. Contraindications to FNAB include inability of the patient to hold the breath, lie immobile on the CT table for more than 30 minutes, or refrain from coughing. Relative contraindications include bleeding diatheses, previous pneumonectomy, severe emphysema, severe hypoxemia, pulmonary artery hypertension, or nodules in which successful biopsy cannot be performed because of their small size or location. FNAB has a sensitivity of 86% and a specificity of 98.8% in the diagnosis of malignancy (24). Sensitivity decreases for nodules 5 to 7 mm in diameter and in patients with lymphoma. When the FNAB sample is interpreted as malignant or if a specific benign condition is diagnosed (Fig. 7.20), further decisions regarding care are dictated by the diagnosis. When a nonspecific benign condition is diagnosed, such as atypical bronchioloalveolar hyperplasia or inflammation without organisms on a smear or a culture, further evaluation with core-needle biopsy or clinical and radiologic follow-up is required. The most common complications of FNAB are pneumothorax and hemorrhage, with pneumothorax occurring in 25% of patients.
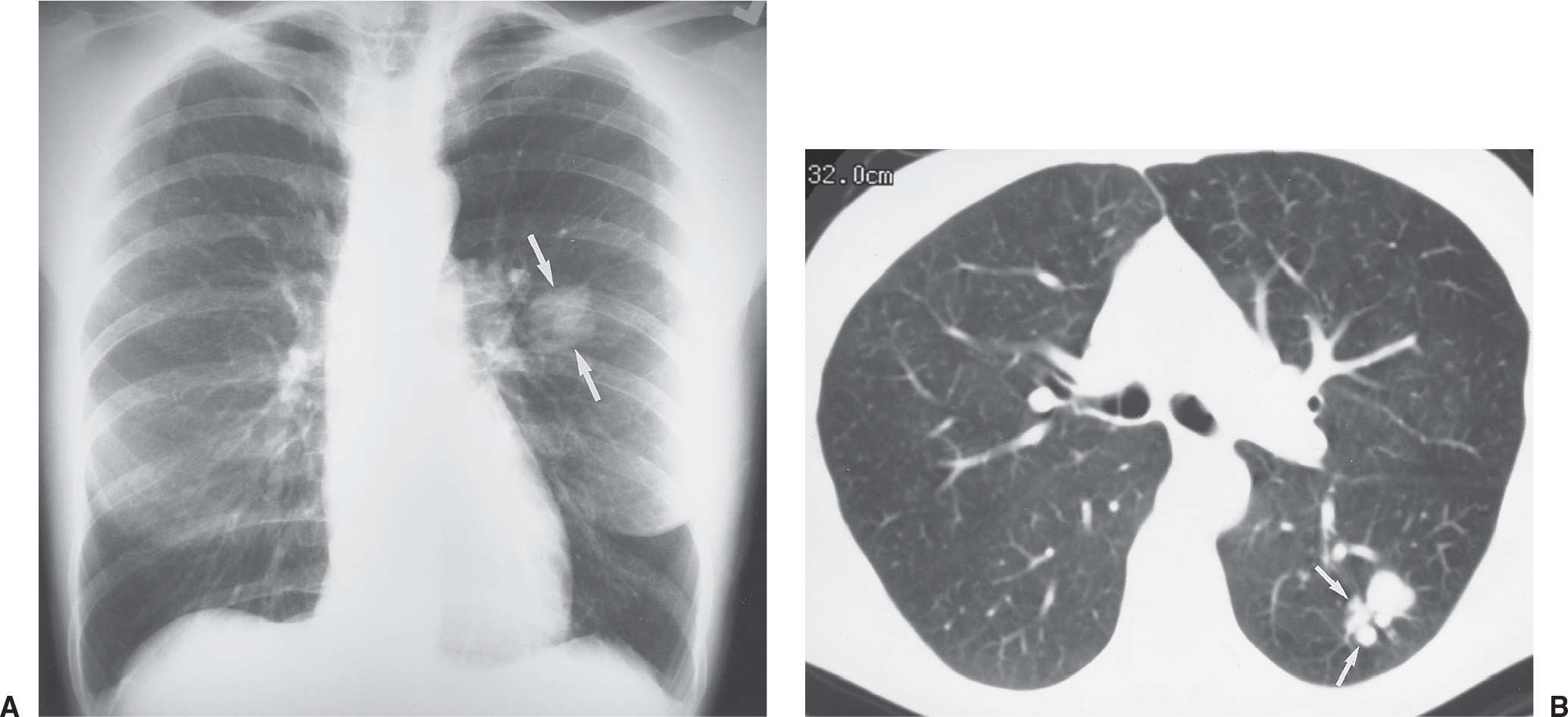
FIG. 7.14 • Coccidioidomycosis. A: PA chest radiograph of a 38-year-old woman living in California with a remote history of pneumonia shows a slightly lobulated, 3-cm nodule in the superior segment of the left lower lobe (arrows), which had enlarged compared with a chest radiograph from 3 months earlier (not shown). B: CT scan shows a dominant nodule, slightly lobulated and notched, and adjacent smaller satellite nodules (arrows). Satellite nodules are more commonly seen with benign entities, but they can be seen with malignant neoplasms also and therefore cannot be used to distinguish benign from malignant nodules.
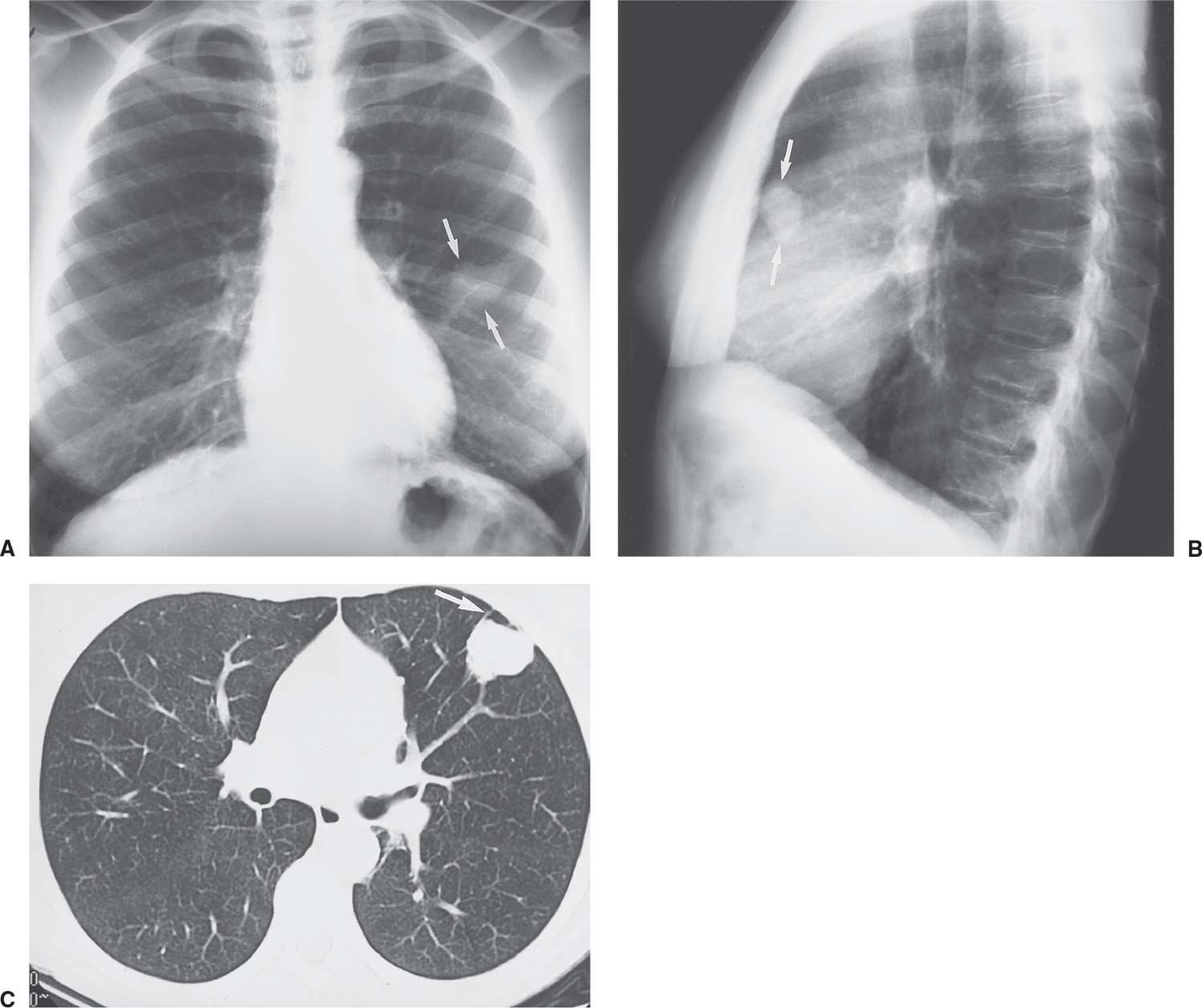
FIG. 7.15 • Large-cell lung cancer. A: PA chest radiograph of a 45-year-old cigarette smoker with a cough for 3 months shows an approximately 3-cm nodule in the left upper lobe (arrows), which was new compared with prior chest radiographs. Note the similarity between the appearance of this nodule and the nodule in Figure 7-14. B: Lateral view confirms the location of the nodule in the anterior left upper lobe (arrows), with no visible calcification. C: CT scan shows that the nodule is slightly lobulated but fairly well circumscribed. The tail sign is present (arrow); this is a nonspecific feature of peripherally located pulmonary lesions that does not distinguish a benign from a malignant lesion.
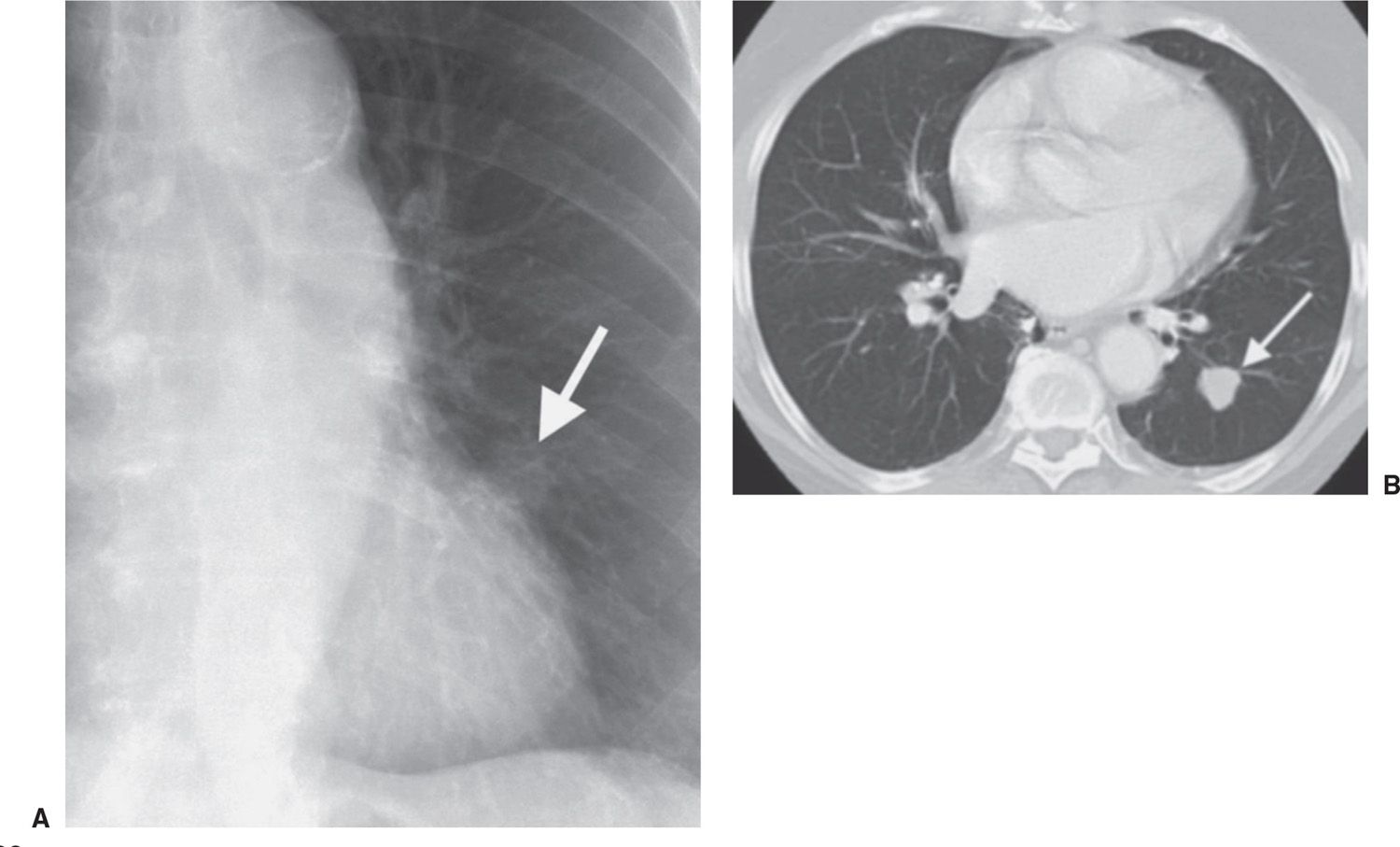
FIG. 7.16 • Non–small-cell lung cancer. A: Chest radiograph shows a subtle nodular opacity adjacent to the left heart border (arrow). B: CT scan shows a slightly lobulated nodule with smooth margins in the left lower lobe (arrow).
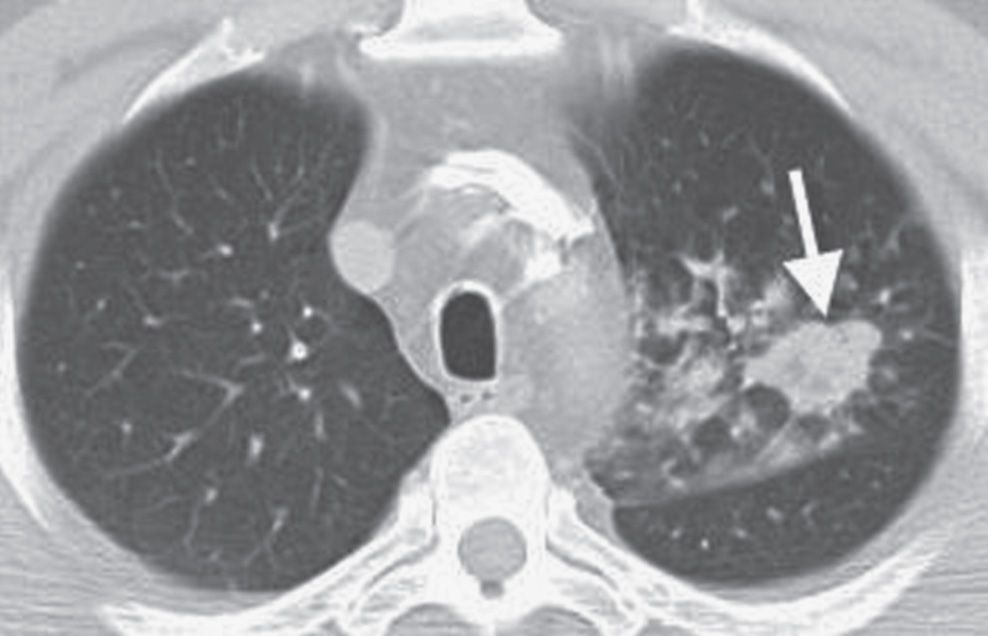
FIG. 7.17 • Primary squamous cell carcinoma. CT scan shows a dominant nodule (arrow) with adjacent smaller irregular nodules and ground-glass opacities.
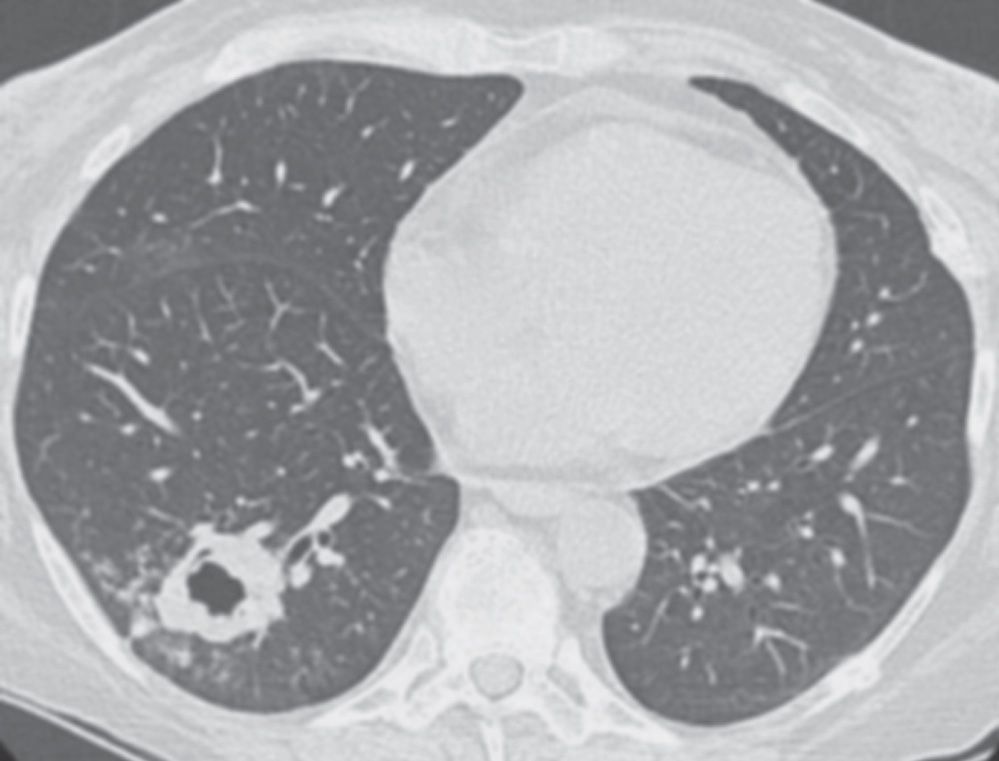
FIG. 7.18 • Invasive pulmonary aspergillosis. CT scan of a 52-year-old woman with a liver transplant shows a thick-walled, irregular, cavitary mass in the right lower lobe.
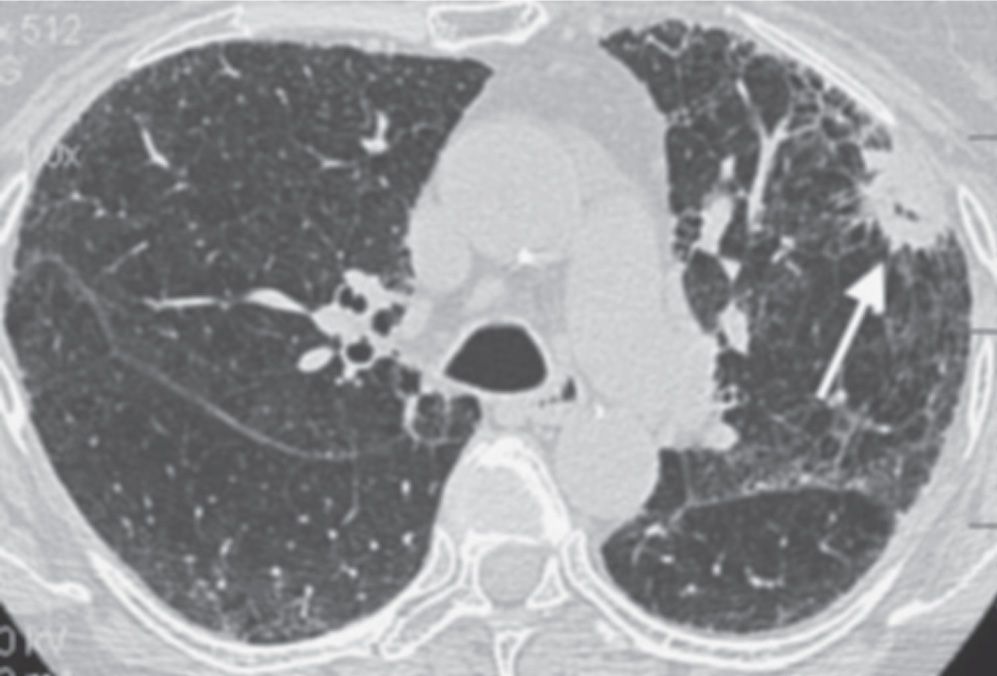
FIG. 7.19 • Primary adenocarcinoma. CT scan of a 66-year-old woman with idiopathic pulmonary fibrosis shows a cavitary spiculated nodule in the left upper lobe (arrow).
The ability to detect very small nodules improves with each new generation of CT scanner. The majority of cigarette smokers who undergo thin-section CT have been found to have small lung nodules, most of which are smaller than 7 mm in diameter (25). Guidelines for follow-up and management of noncalcified nodules detected on nonscreening CT scans were developed before the widespread use of multidetector row CT and still indicate that every indeterminate nodule should be followed with serial CT for a minimum of 2 years. In 2005, the Fleischner Society published guidelines for management of SPNs that are detected incidentally on CT scans (26). The Fleischner Society recommendations apply only to adult patients (35 years of age or older) with nodules that are “incidental in the sense that they are unrelated to known underlying disease.” In patients under age 35, unless they have a known primary cancer, the guidelines suggest that a single low-dose follow-up CT in 6 to 12 months be considered. Patients with a cancer that may be a cause of lung metastases should be cared for according to the relevant protocol or specific clinical situation. An abbreviated set of recommendations for nodule follow-up, based on the Fleischner guidelines, is shown in Table 7.3.
A special class of nodules with a ground-glass component is referred to as “subsolid.” Many subsolid nodules represent peripheral adenocarcinoma, the most common type of lung cancer and a cancer type that is increasing in frequency (27). Guidelines for managing subsolid nodules, based on Fleischner Society recommendations, are outlined in Table 7.4 (27). These guidelines must be interpreted in light of an individual’s clinical history. When evaluating pure ground-glass nodules, regardless of size, a history of extrathoracic malignancy does not preclude following these guidelines. Alternate diagnoses should be considered (e.g., respiratory bronchiolitis in cigarette smokers) in the case of multiple small ground-glass nodules.
MULTIPLE PULMONARY NODULES
The differential diagnosis for multiple pulmonary nodules is different from that for SPNs (Fig. 7.21) (Table 7.5), although there is some overlap. Rheumatoid nodules can be solitary or multiple (Fig. 7.22). In more than 95% of immunocompetent patients with multiple pulmonary nodules, the etiology of the nodules is (a) metastases or (b) infection (typically tuberculous or fungal granulomas) (Fig. 7.23) (2). Determining that the nodules are cavitary is useful in narrowing the list of diagnostic possibilities (Figs. 7.24 to 7.30; Table 7.6) (25). A cavity is defined as a gas-filled space, seen as a lucency or low-attenuation area, within pulmonary consolidation, a mass, or a nodule (1). Those disorders that can result in cavitary nodules can also result in nodules that are not cavitary or that are not appreciated as cavitary on a chest radiograph; therefore, the mnemonic for cavitary nodules, “CAVITY” (Table 7.6), can be remembered as a guide for all cases of multiple pulmonary nodules.
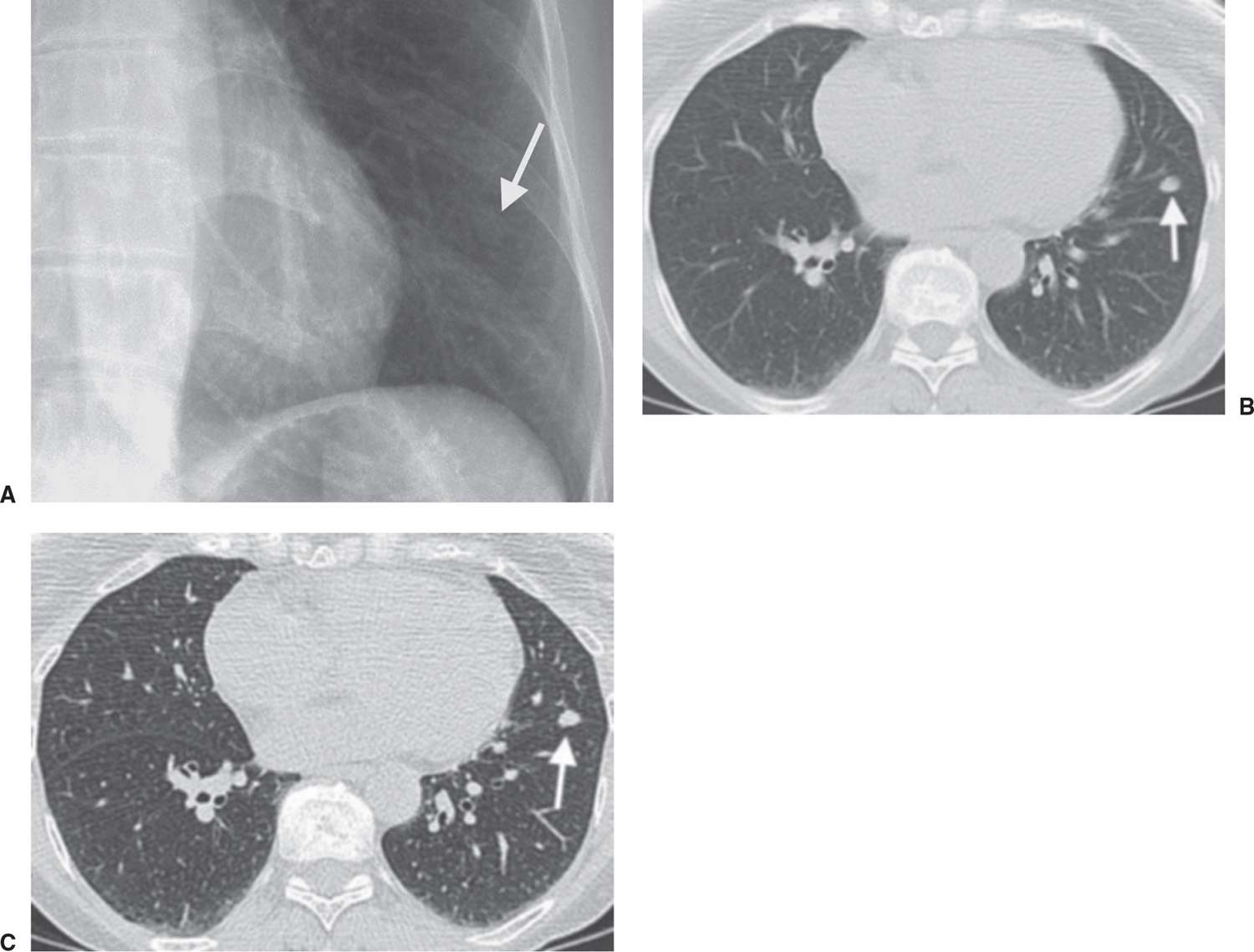
FIG. 7.20 • Benign lymph node. A: Chest radiograph shows a subtle nodule (arrow) in the left lower lobe. B: CT scan (5-mm slice thickness) shows that the nodule has smooth margins (arrow). C: Thin-section CT scan (1.25 mm thickness) shows that the nodule (arrow) is along the left major fissure, consistent with a benign subpleural lymph node. Although the CT findings were highly suggestive of the diagnosis, fine-needle aspiration biopsy was performed, and the diagnosis was confirmed.
Unless the patient is immunocompromised, the great majority of patients who have multiple noncalcified nodules on chest radiographs have metastases. This is even more likely to be the diagnosis when the patient has a known or suspected primary malignancy. The larger and more variable in size that the nodules are, the more likely they are to be neoplastic. Metastases are usually spherical with well-defined margins; as a rule, they vary considerably in size. In autopsy series, the most common sources of metastases from extrathoracic malignancies to the lungs include tumors of the breast, colon (Figs. 7.31 and (7.32), kidney (Fig. 7.33), uterus, prostate, head, and neck (28). Other tumors that have a high incidence of pulmonary metastases, but are not as prevalent in the population and therefore not encountered as frequently, include choriocarcinoma, osteosarcoma, Ewing sarcoma, testicular tumors (Fig. 7.34), melanoma (Figs. 7.35 and (7.36), and thyroid carcinoma. The most common sites of origin of cavitary metastases are the uterine cervix (Fig. 7.37), colon, and head and neck (Fig. 7.38) (29). Squamous cell carcinoma cavitates twice as often as adenocarcinoma (29). Calcification of metastases is seen most commonly with osteosarcoma and chondrosarcoma (Figs. 7.39 and (7.40) or after successful treatment of metastases (30). A miliary nodular pattern of metastases is seen most commonly with thyroid or renal carcinoma, bone sarcoma, trophoblastic disease, or melanoma. On occasion, an SPN will be seen in a patient with a known primary tumor. In a patient over age 35 with a squamous cell cancer elsewhere in the body, the solitary lung lesion is usually a separate primary tumor. If the patient has adenocarcinoma elsewhere, there is an equal chance that the solitary nodule is a primary lung cancer or a solitary metastasis. Cancer of the colon is the most common source of a solitary pulmonary metastasis (Fig. 7.41). If there is a soft tissue or skeletal sarcoma or a melanoma elsewhere, the solitary lung lesion is most often a metastasis (31).
Table 7.3 SUGGESTED RECOMMENDATIONS FOR FOLLOW-UP AND MANAGEMENT OF SOLID NODULES DETECTED INCIDENTALLY ON CT IN PATIENTS 35 YEARS OF AGE OR OLDER WITHOUT KNOWN CANCER
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree