Summary of Key Points
- •
Transvaginal sonography (TVS) provides valuable information as part of the initial evaluation of an infertile patient.
- •
EVS has an essential role in monitoring endometrial thickness and morphology as well as follicular development during hormonal stimulation.
- •
EVS or transabdominal sonography provides guidance for oocyte retrieval.
- •
Postretrieval complications such as ovarian hyperstimulation, hemorrhage, and infection are optimally imaged with sonography.
Infertility can be defined as the inability to conceive a pregnancy after 1 year of unprotected intercourse or after 6 months in a woman over 35 years old. It affects between 6% and 10% of couples in the United States. Because fertility peaks in the third decade and subsequently declines, the age of the female partner is an important variable in the treatment of infertility. The use of sonography, in particular EVS, has become an integral component of the evaluation and treatment of infertility. The transvaginal approach allows high-resolution assessment of the uterus, ovaries, and fallopian tubes. EVS plays a critical role in the diagnosis and treatment of infertile women in combination with serologic testing, physical examination, and careful assessment of medical and surgical history, as well as evaluation of the male partner. Initial baseline ultrasound examination is used primarily to identify structural abnormalities that might affect fertility such as uterine anomalies, endometrial polyps or submucosal leiomyomas, endometrial adhesions/synechiae, or hydrosalpinges. Sonography is also used to assess for possible underlying pathologic processes associated with infertility such as adenomyosis, endometriosis, polycystic ovary syndrome (PCOS), and low antral follicular count. If the baseline pelvic sonogram is inconclusive or noncontributory, further anatomic evaluation can be obtained by means of pelvic magnetic resonance imaging (MRI), hysterography, sonohysterography, or even hysteroscopy and laparoscopy, as indicated. It is estimated that ovulatory defects are the primary cause of infertility in 20% to 40% of infertile women. Structural abnormalities of the female reproductive tract account for approximately 30% of cases; male factors up to 35%; and cervical mucosal, peritoneal, or unexplained abnormalities account for approximately 10% to 15%. Once a treatment plan has been established, sonography plays an important role in monitoring response, particularly in assessing folliculogenesis and endometrial receptivity. In addition, ultrasound imaging is crucial for guiding infertility treatment such as oocyte retrieval and in assessing posttreatment complications. This chapter will review the role of sonographic imaging in the diagnosis and treatment of women presenting with infertility.
Initial Evaluation
Part of the initial evaluation of all women with infertility is an assessment of pelvic anatomy. Although high-resolution EVS is the preferred method for imaging the pelvic organs, an initial overview using a transabdominal approach should be performed to evaluate for the possible presence of an enlarged uterus or other pelvic mass. Transvaginal probes are higher frequency than transabdominal transducers and, thus, provide high-resolution images of the uterus, ovaries, and fallopian tubes, but with the tradeoff of reduced penetration and smaller field of view. Masses that extend out of the pelvis cannot be fully imaged or characterized with the transvaginal approach, as they extend beyond the field of view of the transducer. A formal transabdominal sonogram, performed with a distended urinary bladder, or a combination of both transabdominal and transvaginal imaging may be required in some patients. A baseline transvaginal sonographic examination consists of images of the uterus, endometrium, and ovaries, with additional targeted evaluation of any identified abnormality. The reader is referred to individual chapters elsewhere in this textbook about the uterus ( Chapter 28 ), ovaries ( Chapter 30 ), and fallopian tubes ( Chapter 31 ) for additional detail.
Initial Evaluation
Part of the initial evaluation of all women with infertility is an assessment of pelvic anatomy. Although high-resolution EVS is the preferred method for imaging the pelvic organs, an initial overview using a transabdominal approach should be performed to evaluate for the possible presence of an enlarged uterus or other pelvic mass. Transvaginal probes are higher frequency than transabdominal transducers and, thus, provide high-resolution images of the uterus, ovaries, and fallopian tubes, but with the tradeoff of reduced penetration and smaller field of view. Masses that extend out of the pelvis cannot be fully imaged or characterized with the transvaginal approach, as they extend beyond the field of view of the transducer. A formal transabdominal sonogram, performed with a distended urinary bladder, or a combination of both transabdominal and transvaginal imaging may be required in some patients. A baseline transvaginal sonographic examination consists of images of the uterus, endometrium, and ovaries, with additional targeted evaluation of any identified abnormality. The reader is referred to individual chapters elsewhere in this textbook about the uterus ( Chapter 28 ), ovaries ( Chapter 30 ), and fallopian tubes ( Chapter 31 ) for additional detail.
Baseline Evaluation
Uterus
Imaging of a patient with infertility often begins with hysterosalpingography (HSG) to assess for uterine anomalies and determine tubal patency. Sonography, including three-dimensional (3D) coronal views, is also a highly accurate initial imaging modality to evaluate for possible structural abnormalities such as congenital uterine anomalies, masses, or adenomyosis and is an integral part of the baseline evaluation. At real-time sonography, the uterus should be imaged in both longitudinal (sagittal) and coronal (or transverse) orthogonal planes, with some views including the full length of the cervix. In patients with an enlarged or elongated uterus, it is important to ensure that the entire fundus as well as any exophytic lesion is included. The normal uterus is oval in shape with a curved, slightly convex fundal contour. Uterine malformations are reported to occur in 1% to 7% of infertile patients. These malformations are often initially identified at HSG but can be appreciated on the baseline ultrasound image in most affected patients.
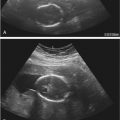
A uterine anomaly may be suspected at real-time sonography when, in the transverse plane, the endometrium appears to separate toward the fundus. In this instance, the addition of a 3D coronal view, which provides direct visualization of the fundal contour, the shape of the endometrial cavity, and characterization of any septum between the two uterine horns, can be extremely helpful in categorizing the type of uterine anomaly, which can range from an arcuate configuration (considered by most to be within the range of normal) to uterine didelphys ( Fig. 32-1A and B ). If a uterine anomaly is suspected but cannot be confirmed, repeating the 2D and 3D sonography during the secretory phase of the menstrual cycle, when the endometrium is thick and hyperechoic and thus more visible, may be beneficial. If concern for a uterine anomaly and need for characterization persists, further imaging with pelvic MRI may be helpful. In addition, MRI may provide additional important structural information in patients with complex anomalies ( Chapter 36 ).

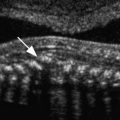
Another major component of the baseline sonogram is evaluation of the endometrial pattern and thickness, typically assessed on the midline sagittal long-axis view. The endometrial morphologic appearance and thickness change through the menstrual cycle in response to rising serum estrogen concentrations. During the menstrual phase, the endometrium appears thin, linear, regular, and homogeneously echogenic, typically measuring less than 5 mm in thickness ( Fig. 32-2 ), although in some patients, a more heterogeneous pattern can be seen. Occasionally a small amount of fluid is present in the uterine cavity. This fluid is not included in the reported measurement. Rather, the two endometrial layers (anterior and posterior) are measured separately and added together for reporting purposes ( Fig. 32-3 ). At the baseline study, careful evaluation of the endometrium is necessary to search for intracavitary lesions such as submucosal leiomyomas or endometrial polyps ( Fig. 32-4A and B ). Focal lesions in the endometrial cavity are typically removed prior to fertility treatment, as resection has been reported to be associated with increased pregnancy rates. In a retrospective study of infertility patients with endometriosis published in 2011, Shen and associates reported a clinical pregnancy rate of 49.5% in patients following hysteroscopic polypectomy compared to a pregnancy rate of 29.8% in those without polyps. Medina and colleagues reported in a randomized, prospective study that infertility patients with endometrial polyps undergoing intrauterine insemination were twice as likely to become pregnant if hysteroscopic polypectomy was performed prior to insemination, with a relative risk of 2.1 and a pregnancy rate of 59% in the study group versus 25.4% in the control group. Rarely, other more unusual types of endometrial diseases, such as unsuspected retained products of conception or osseous metaplasia ( Fig. 32-5 ), are found on baseline imaging; these lesions should also be addressed prior to initiation of fertility treatment. In some patients, sonohysterography may provide further information regarding endometrial disease and is particularly useful in identifying adhesions within the endometrial cavity (Asherman syndrome). Asherman syndrome most commonly occurs following instrumentation such as dilatation and curettage (D&C) but can also occur following intrauterine device placement, uterine artery embolization, radiation therapy, pregnancy, or endometritis. Up to 43% of women with Asherman syndrome will have difficulty conceiving or will present with recurrent early pregnancy loss. Sonohysterography may also be helpful in identifying and delineating submucosal leiomyomas and polyps ( Fig. 32-6 ).





The myometrium is also evaluated on the baseline sonogram. Abnormalities of the myometrium that may affect fertility include adenomyosis and leiomyomas (also called fibroids ). Adenomyosis occurs when endometrial glands and stroma burrow into the subjacent myometrium. The classic appearance on EVS is a globular, smooth-contoured, enlarged uterus with asymmetric wall thickening, heterogeneity of the myometrium, thickening of the subendometrial halo, subendometrial myometrial cysts or echogenic nodules or striations, and pencil-thin dark linear radiating striations through the myometrium ( Fig. 32-7 ). The presence of adenomyosis is associated with a significantly lower clinical pregnancy rate, with clinical pregnancy defined as a pregnancy documented by visualization of a gestational sac or embryo, whereas chemical pregnancy indicates a pregnancy diagnosed by documentation of a positive human chorionic gonadotropin (hCG) level. The cause of infertility in patients with adenomyosis is not fully understood but may be related to decreased myometrial contractility, which is necessary to transport the sperm and zygote through the uterus. In addition, adenomyosis has a strong association with endometriosis, particularly in patients less than 36 years of age. Patients with adenomyosis also have an increased risk of early pregnancy loss as compared to patients without adenomyosis.

Myomas are found in approximately 20% to 40% of women, with the rate increasing with patient age. The percentage of infertile patients with myomas will likewise vary with age. The classic sonographic appearance of a myoma is a solid mass with well-defined borders. Echogenicity is variable, with most being hypoechoic. Larger myomas are often heterogeneous and may demonstrate a typical swirling pattern, edge refraction, posterior shadowing, and focal areas of calcification on EVS. Identifying and documenting the location of all myomas, particularly in relation to the endometrial cavity and lower uterine segment, are important components of the baseline ultrasound examination. Myomas that deviate or protrude into the endometrial cavity (submucosal) ( Fig. 32-8A and B ) appear to have a deleterious effect on fertility because of interference with implantation, possibly secondary to pressure effect on the endometrium, which decreases receptivity, and may cause mechanical blockage of the endocervical canal or fallopian tubes. Hence, the possible presence of submucosal myomas should be carefully assessed during the preliminary workup of the infertile patient. An increased rate of first trimester pregnancy loss is also found in patients with submucosal myomas, and resection of submucosal myomas appears to enhance fertility. Although the presence of subserosal myomas does not appear to adversely affect conception rates, the effect of intramural myomas on fertility remains unclear. It has been proposed that the presence of intramural myomas adversely affects uterine peristalsis, with more frequent peristalsis associated with poor pregnancy rates. Patients with intramural myomas also appear to have higher miscarriage rates. However, it is not clear whether resection of intramural myomas is beneficial. Because of all this, careful documentation with precise localization of all uterine myomas in the infertile woman is extremely important. Three-dimensional sonography can be helpful in delineation of centrally positioned myomas ( Fig. 32-9A and B ). If myoma location remains indeterminate, MRI, saline infusion sonohysterography (SIS), HSG, or hysteroscopy should be considered, as these techniques provide the most accurate evaluation of the endometrial cavity and demonstration of possible submucosal myomas. Although myomectomy is the typical treatment option, for some patients with multiple large myomas for whom myomectomy is not an option, uterine artery embolization may have a role (see Chapter 37 ).


Ovary
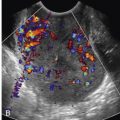
On baseline imaging, the ovaries are routinely evaluated to assess for normal expected findings and to screen for any abnormality. In the first portion of the menstrual cycle, a normal ovary will demonstrate multiple antral follicles, which measure between 2 and 9 mm in maximum diameter ( Fig. 32-10 ). The antral follicle count (AFC) can be correlated with fertility status, response to ovarian stimulation, and success of conception. A low AFC of 4 to 10 follicles between days 2 and 4 of a regular menstrual cycle suggests poor ovarian reserve, which assists in predicting outcome for fertility treatments. Infertile patients over 35 years of age, patients with a single ovary, a prior history of pelvic radiation therapy or chemotherapy, and patients with a family history of early menopause are at increased risk of decreased ovarian reserve. The potential role of 3D sonography in assessing the AFC, aiming to allow for remote evaluation of the ovary via stored 3D imaging, is under investigation. Focal ovarian lesions are frequently found at the baseline ultrasound examination. The most common is the residual corpus luteum, a complex thick-walled cyst related to ovulation during the prior menstrual cycle ( Fig. 32-11A and B ). This is a normal physiologic process, and this “cyst” will spontaneously involute. Additional lesions that might be identified at baseline EVS include endometriomas ( Fig. 32-12 ) and benign ovarian tumors such as dermoid cysts. The ovaries should be assessed for sonographic findings suggestive of PCOS. Patients with PCOS often present with the clinical triad of amenorrhea, hirsutism, and obesity, although clinical presentation is variable. Patients with PCOS are at increased risk of anovulation and thus infertility. In 2003, the Rotterdam Consensus workshop published what was considered the standard sonographic diagnostic criteria for PCOS: 12 or more 2- to 9-mm follicles in each ovary and/or increased ovarian volume measuring more than 10 mL ( Fig. 32-13 ). More recently, it has been noted that with improved image quality and resolution resulting from rapid technologic advancements in sonographic equipment, more than 12 tiny follicles are often seen in healthy women with normal ovaries. Therefore, adhering to the Rotterdam criteria could potentially lead to an overdiagnosis of PCOS. A modification of the Rotterdam criteria has been suggested with a threshold of over 25 small follicles necessary for the diagnosis of PCOS in patients imaged with state-of-the-art, high-resolution sonographic equipment. At present, this issue has not been settled. The imager is reminded that PCOS is a clinical diagnosis and the sonographic findings should not be used in isolation, as they are neither entirely sensitive nor specific.