Abstract
Two-dimensional speckle tracking echocardiography (2DSTE) is a non-Doppler technique that applies computer software analysis of images generated by conventional ultrasound techniques to calculate parameters of motion (displacement, velocity) and deformation (strain, strain rate) in all three axes and thus providing a comprehensive assessment of cardiac function. This chapter will describe the basic concepts and terminology of 2DSTE, optimal image acquisition and image processing steps, a guide to interpretation of the results, and reference values for term and preterm infants.
There are well-established clinical applications of 2DSTE in adults and a growing amount of studies describing its clinical use in newborns. 2DSTE is more sensitive compared with conventional echocardiography parameters in detecting functional changes during acute hypoxia, with high fetal insulin levels (infants of diabetic mothers) or after chronic hypoxia (small-for-gestational age infants). 2DSTE can help to detail changes in loading conditions, as seen in preterm infants with a patent ductus arteriosus and/or bronchopulmonary dysplasia, and can be used for longitudinal assessment of (corrected) congenital heart disease. We expect this area of research to expand rapidly, as it did in adult cardiology. 2DSTE has now been thoroughly tested in term and preterm infants, often better compared with commonly used conventional parameters, and is anticipated to find a place in updated recommendations for use in clinical practice.
Keywords
newborn, preterm infant, reference values, speckle tracking echocardiography, ventricular function
- •
Two-dimensional speckle tracking echocardiography (2DSTE) is a non-Doppler technique that applies computer software analysis of images generated by conventional ultrasound techniques.
- •
2DSTE calculates parameters of motion (displacement, velocity) and deformation (strain, strain rate) in all three axes.
- •
Advantages of 2DSTE are its relative angle independency, the option for retrospective analysis, and most parameters of interest are provided from a few selected views.
- •
As with any ultrasound technique, image quality is the most important aspect to optimize feasibility and reliability.
- •
Reliability is superior compared with current conventional parameters, and reference values have been established in the term and preterm population.
- •
2DSTE and tissue Doppler studies have significantly increased our knowledge of systolic and diastolic function and its development in preterm infants.
- •
Deformation parameters are more sensitive in detecting abnormalities in specific newborn populations, such as severe growth restriction, maternal diabetes, hypoxic ischemic encephalopathy, and congenital heart disease.
- •
Intervendor and intravendor variation in algorithms used in the 2DSTE software limits direct comparison of results.
Echocardiography is the most commonly used diagnostic modality for cardiovascular assessment in the newborn. It is a noninvasive bedside method to study cardiac structure and provide an estimate of cardiac function. However, measuring the true nature of cardiac mechanics is difficult due to the complex myocardial geometry and how the determinants of cardiac function (i.e., preload, contractility, afterload) interact with each other.
The developing heart starts out as an isotropic tissue built of cardiomyocytes and supportive tissues and gradually develops into an anisotropic tissue where groups of cardiomyocytes are structured as laminar sheets of fibers. In the subendocardial region the fibers are longitudinally oriented along the axis of the heart, with an angle to the right (right-handed helix), in the midwall the fibers are circumferentially orientated, and in the subepicardial region they are longitudinal again but with an angle to the left (left-handed helix; Fig. 13.1 ). This double helical structure of the heart is important for its function. Being able to shorten in longitudinal and circumferential directions simultaneously and thus creating a twisting motion greatly improves energy efficiency and the ability to empty and fill the heart with blood. In the adult heart a 60% ejection fraction (EF) can be achieved with only 15% fiber shortening.

Ventricular motion thus consists of narrowing, shortening, lengthening, widening, and twisting. Furthermore, as the myocardium moves and changes its position, it will undergo deformation and change its shape because not all parts move with the same velocity. Because the myocardial fibers are not compressible, any shortening in one direction leads to expansion in other directions. Part of this will be actual deformation, but wall thickening is greater than the sum of the individual fiber thickenings due to longitudinal fibers shifting inward (shearing).
Not all aspects of cardiac mechanics can be captured using conventional echocardiography techniques. It is difficult to capture the twisting motion or to capture wall thickening for the whole ventricle. Furthermore, wall motion measurements cannot differentiate between active and passive movement of a myocardial segment. On conventional echocardiography, a myocardial segment which has lost its function will still show movement due to the tethering effect of adjacent segments. Deformation analysis is an approach in which the limitations of conventional techniques are minimized. Deformation analysis is possible using invasive sonomicrometry, magnetic resonance imaging (MRI), tissue Doppler imaging (TDI), and a novel technique called speckle tracking echocardiography. This chapter will introduce the reader to two-dimensional speckle tracking echocardiography (2DSTE) of the left ventricle (LV) and right ventricle (RV). We will discuss some basic concepts, terminology, the practicalities of hardware setup and image acquisition, and its current status in newborn infants. For in-depth technical aspects of ultrasound in general and deformation imaging with 2DSTE in adults, we recommend the following website: http://folk.ntnu.no/stoylen/strainrate/ .
Basic Concepts and Terminology
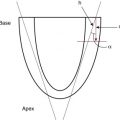
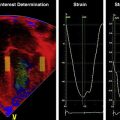
Speckle tracking echocardiography is a non-Doppler technique that applies computer software analysis of images generated by conventional ultrasound techniques. The Doppler ultrasound signal generates artifacts due to random reflections, called speckles. These speckles stay stable during the cardiac cycle and can act as natural acoustic markers. Speckle tracking software can define and follow a cluster of speckles from frame to frame to calculate parameters of motion (displacement and velocity) and parameters of deformation (referred to as strain and strain rate [SR]). Strain is the percentage of change from its original length, with negative values describing narrowing or shortening and positive values lengthening or thickening. SR is the average change in strain per unit of time and is expressed as 1/s. Motion and deformation are three dimensional (3D) and described along the three cardiac axes. The nomenclature is longitudinal for base-to-apex, circumferential for rotational, and radial or transverse for inward motion and deformation ( Fig. 13.2 ). The difference in the rotation of the myocardium between the apical and basal short axis plane is commonly referred to as twist and reported in degrees. If rotation is normalized to the distance between the respective image planes, it is referred to as torsion and reported in degrees per centimeter. Normalizing twist to LV length facilitates comparison of LV rotational mechanics across differing age groups, but this can only be done accurately with 3D imaging.

Recommended nomenclature, units, and abbreviations for 2DSTE-derived parameters are provided in an important consensus report. The report also provides consensus on timing of mechanical events, essential for standardization of almost all derived parameters of motion and deformation. To be able to report on Lagrangian strain (where the original length is known), the software would need a reference point in time (the beginning of the cardiac cycle) and an end point where deformation is determined according to its reference point. End diastole is commonly taken at the beginning of the cardiac cycle, either determined from the four-chamber images as the frame before the mitral valve completely closes or using surrogates such as the R peak of the electrocardiogram (ECG) or the largest volume of the LV. End systole is the usual end point and coincides with aorta valve closure, determined from apical long axis views or using surrogates such as the end of the T wave of the ECG, the end of the negative spike after ejection on the 2DSTE-derived velocity trace, or the minimum volume of the LV. The current consensus in adult cardiology is to report on end systolic strain, with other parameters reported in addition ( Fig. 13.3 ).

Most studies of newborns reported on peak systolic strain (the first peak strain value found) or maximum systolic strain (the highest strain value found). Postsystolic strain has not been reported in newborns but could prove to be an important parameter in fetal growth restriction.
Because the heart contains layers of fibers in different directions, motion and deformation can vary depending on where in the myocardial wall they are measured (i.e., at the endocardium, midwall, or averaged over the entire cardiac wall). Whether this also plays a role in the analysis of the thin walled and immature myocardium of newborn infants is unclear. Older software versions generally report on endocardial deformation, whereas newer versions report on (the lower) global or midwall deformation with separate reporting for each wall area.
To help interpret the data, it is important to understand the difference between global and segmental motion and deformation. The term global deformation should be reserved for the combined findings from the four chamber, two chamber, and long axis apical views or from the basal level of the papillary muscle and apical short axis views. Segmental deformation can be vendor specific because the segmental model used could describe 16, 17, or 18 segments. Most software packages would divide the ventricular wall into six equidistant segments termed basal, mid, and apical segments for the apical views and anatomically for the short axis views (i.e., anteroseptal, anterior, lateral, posterior, inferior, and septal wall segment). Data can be reported as global (all segments of all views), for the four chamber view alone (six segments), per ventricular wall (three segments of respectively the LV free wall, RV free wall, and the septum), or per individual segments (i.e., basal segments only for 2DSTE-derived myocardial velocities). Most 2DSTE data to date in newborns have been determined from the apical four-chamber view alone or per free wall because not all software can calculate global parameters when heart rate varies too much between the different views.
Image Acquisition
Speckle tracking analysis can be performed on normal 2D gray-scale images but does require some adjustments to optimize feasibility of successful analysis. As mentioned earlier, 2DSTE tracks artifacts that are there because of random reflections of the Doppler signal; hence the optimal image should contain a combination of a perfect 2D gray-scale image with enough artifacts (speckles) within the myocardial wall. This is not always easy to achieve. The feasibility of 2DSTE analysis in newborns has been reported between 70% and 95% of the images acquired, with the highest feasibility in the more recent studies. As with any ultrasound imaging, the poorer the image quality, the less useful is the analysis. Images selected for analysis should be reviewed for image quality before analysis is attempted. Using a subjective or objective image quality scoring sheet can assist in increasing the feasibility of 2DSTE analysis. Quality items include completeness of the ventricle, how the endocardial and epicardial borders appear, the presence of artifacts, the ECG trace, and frame rate used ( Table 13.1 ).
| Quality Item | Description |
|---|---|
| Ventricle shape | No chamber foreshortening (apical views) Symmetry of the basal segments and valve (apical views) Contains circular images, not oblique cuts with ellipsoid shape (short axis views) |
| Endocardial and epicardial borders | Enough speckles within each segment of interest Clear view of the endocardial and epicardial borders of all segments throughout the cardiac cycle |
| Gain settings | Overall gain and image settings Distribution of gain over the myocardial segments |
| Artifacts | Reverberation, drop outs, breathing motion |
| Frame rate | Optimized to 0.7–0.9 frames/sec/bpm |
| ECG signal | Clear R wave |
For optimal clarity of the endocardial borders, the overall gain setting and time-gain compensation should be adjusted to produce images that appear brighter than those typically used in conventional echocardiography. Newborns are often not sedated for image acquisition, so motion and breathing can cause some segments to move in and out of the plane of view.
Reproducibility of newborn deformation parameters were most robust when images were obtained with a frame rate to heart rate ratio between 0.7 and 0.9. Low frame rate can cause undersampling and thus underestimate peak systolic SR values. Strain is less frame rate sensitive because the rate of change is lowest at end systole. The operator can adjust depth and sector width to optimize frame rate, but reaching optimal frame rates might still be a challenge in newborns with very high heart rate and will require high-end ultrasound machines.
A clear ECG signal is important for the speckle tracking software to allow proper gating of the images (i.e., capture two to four cardiac beats triggered by the R wave). On most modern equipment, the ECG trace can easily be obtained by connecting the ultrasound machine to the neonatal monitor without the need for machine-specific ECG leads.
Although 2DSTE as a technique is angle independent, the angle of insonation can still make a small difference in strain and SR. This could be explained by differences in image quality and the appearance of the speckles under different angles. Parallel insonification of some segments of the apical views or the anterior and inferior-septal segments of the short axes images can lead to a significant reduction of speckles due to the fiber orientation ( Fig. 13.4 ).

Sending and Storage of Acquired Images
After image acquisition, most operators would send and store selected images for offline analysis on a dedicated workstation. It is crucial to check the send and storage settings on the ultrasound machine before deformation imaging is started. The default setting for most commercial ultrasound machines is to send all images in compressed format Digital Imaging and Communications in Medicine (DICOM) at a frame rate of 30 Hz. Some ultrasound machines now include a separate acquire button to send selected images in RAW format, but these images can only be analyzed using vendor-specific software. For vendor-independent software that uses DICOM images, the send and storage setting of the machine should be changed to “acquired frame rate.” This setting will significantly increase the need for server storage space and might cause network congestion, depending on your local situation.
Image Processing
Postprocessing or the actual speckle tracking analysis is a semiautomated process of selecting a clip with optimal image quality and tracing the myocardial wall slightly within the endocardial border (or other areas of interest) as a sequence of points on a single frame. The software then generates a region of interest (ROI) to include the entire myocardial thickness. It is important to standardize settings for the width of the ROI, if available. There is a learning curve for operators new to the technique to optimize imaging of the entire endocardial wall throughout the cardiac cycle and provide reproducible point selection for tracing.
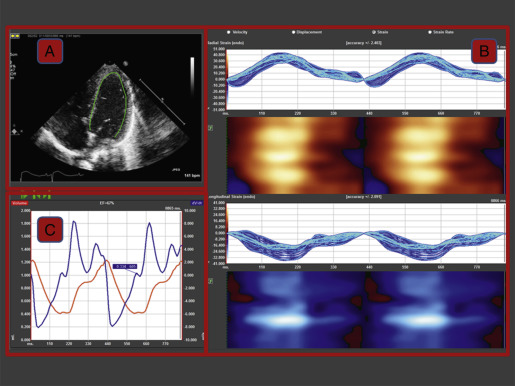
The software will track and trace the sequence of points from frame to frame throughout the cardiac cycle and renders a volume curve and segmental, average, and global curves for velocity, displacement, strain, and SR ( Fig. 13.5 ). After the first analysis, the operator should perform a visual frame-by-frame analysis of tracking accuracy by reviewing the underlying image loop with the superimposed tracking results. Some analysis software offers an automated measure of tracking accuracy, but it is recommended to always perform an operator check, and if needed, adjust the ROI.
Interpretation of the Results
Most software will have the option to present many parameters of interest, but this chapter will focus on the deformation parameters. Strain is measured at end systole and thus at the end of ventricular contractile performance and is closely related to stroke volume (SV) in healthy hearts. SR is recorded earlier in the cardiac cycle and thus will not be subject to load during the entirety of systole. SR is measured at peak ventricular performance and less load dependent, and thus SR is considered to be closely related to myocardial contractility.
The vendor’s software package will determine how the results are presented, but most packages would show the data as color-coded parametric images, systolic numerical values, and as time curves (see Fig. 13.5B ). The patterns of strain should be reviewed by exploring base-to-apex and left-to-right gradients. In newborns, LV apical strain and SR are usually higher compared with the mid and the basal segments, and the reverse is true for displacement and velocity. Deformation in the septum is usually lower compared with both the RV and LV free wall. Segmental abnormalities, as seen after myocardial infarcts, are rare in newborns.
The curves should also be reviewed for diastolic events, best appreciated in the basal segments for 2DSTE-derived myocardial velocities and in the apex for strain and SR. The reliability of diastolic 2DSTE parameters is not optimal, possibly due to the relative low frame rate per heart beat as compared with TDI. Another issue with diastolic events in newborns is that fusion of the early and late diastolic events (EA fusion) commonly occurs at high heart rates (>170/min). Such images would have to be omitted from analysis, prompting much data to be lost. It is not always clear how newborn investigators handled this issue. One study in preterm infants reported all fusion events as late diastolic events instead of omitting the data.
Circumferential motion and deformation and rotational mechanics are derived from the short axis images. Cardiac mechanical dysfunction is usually present in all directions in newborns, but under certain circumstances rotational deformation can be a compensatory mechanism and provide clues for early detection of disease.
All software also reports on radial and transverse deformation. The reliability for these parameters in newborns has been poor, possibly due to the small area changes, and is not routinely reported in newborn studies.
Cantinotti et al. suggested that deformation parameters should be normalized to age and body size. We, and others, do not think that this is necessary for strain and SR. Deformation parameters are fractional changes in length of a cardiac segment relative to its original length, and thus values are already normalized for cardiac size. In adult hearts, there is a clear relationship between cardiac size, SV, and deformation parameters, with a dilated heart able to generate a larger SV with the same contractile force. Only when the observed changes deviate from the predicted changes is myocardial contractility then reduced. We could replicate some of these findings in a cohort of preterm infants with volume load due to a patent ductus arteriosus (PDA), suggesting that cardiac remodeling and subsequent mechanical changes are already present from very early cardiac developmental stages.
Deformation parameters are load dependent and should be interpreted in the context of existing or changing loading conditions. Strain and SR are positively influenced by preload and negatively by afterload. In a meta-analysis of normal ranges of longitudinal strain (S L ) in adult studies, systolic blood pressure (an important determinant of LV afterload) at the time of the study was associated with normal variation and should be considered in the interpretation of strain.
Advantages of Two-Dimensional Speckle Tracking Echocardiography
In an expert consensus paper, Mor-Avi et al. described 2DSTE as “a significant contribution to the much needed process of the transformation of echocardiography from a subjective art of image interpretation to a set of objective diagnostic tools.” When a good-quality image is acquired, the process of 2DSTE is fairly easy and reproducible with postprocessing times reduced to acceptable levels with the ever-increasing advancements in computer processing. As the process is computerized, it takes away some of the subjective human components of analysis. 2DSTE can obtain most parameters of interest (motion, deformation, volume) from a few selected views, with SR being the closest echocardiographic parameter to measure actual contractility. As such, 2DSTE provides incremental value in the assessment of cardiac mechanics and cardiac function.
2DSTE offers minimal angle dependency, as opposed to TDI or conventional measurements. Aligning the probe to reduce the angle of insonation to less than 30 degrees can be difficult under specific situations. For example, preterm infants with volume overload have a more spherical shape of the LV, thus increasing the angle between the probe and the basal segment of the LV free wall. The same can occur in the RV in infants with severe pulmonary hypertension.
2DSTE allows for retrospective analysis of acquired images during specific unexpected events. Our group recently analyzed cardiac mechanics during bradycardia events in preterm infants and was able to retrospectively provide a full set of cardiac function parameters from beat to beat. Systolic function and SV were maintained but cardiac output reduced proportionally to the decrease in heart rate.
Although not its primary purpose, 2DSTE can semiautomatically determine LV volumes. SV is the most common parameter used in newborn echocardiography to describe cardiovascular function. We assessed the agreement between manual biplane SV, semiautomated biplane SV using 2DSTE, and SV using the velocity time integral (Vti) method in 50 preterm infants. Manual and semiautomated SV showed good agreement, but both were significantly lower compared with SV using the Vti method. Further development of this application has the potential to increase the reliability of SV measurements in newborn infants.
Limitations and Pitfalls of Two-Dimensional Speckle Tracking Echocardiography
Although 2DSTE is a relatively easy process, this can also be its problem. The software will render curves and output, sometimes irrespective of basic image issues such as foreshortening or Doppler artifacts, and it remains up to the experience of the operator to determine if the output is valid. Because true cardiac motion is 3D, speckles might move out of the 2D plane and can thus not always be tracked correctly using a 2D technique.
2DSTE has been validated in animal and human studies against sonomicrometry and tagged MRI. However, in newborns and particularly in preterm infants, there is no clear noninvasive gold standard, as even MRI struggles with acquiring images in high resolution at very short time intervals. Hence, the true accuracy of the technique has not been established in newborns.
Currently the most important issue with 2DSTE is the fact that each vendor has its own software algorithm to calculate motion and deformation parameters. This has led to significant intervendor variation and is thus limiting the establishment of 2DSTE as a technique for everyday practice. However, in a joint venture with the industry, further standardizations and upgrades of existing software algorithms were able to improve interobserver and intraobserver reproducibility of global S L in adults. The reliability of 2DSTE is now comparable with or superior to that of EF and other conventional echocardiographic parameters. We are not sure if these findings can be extrapolated to the analysis of the small hearts of preterm infants because the most recent versions of Qlab and Tomtec still showed significant differences in S L .
Normal Two-Dimensional Speckle Tracking Echocardiography Values in Newborns
In the healthy developing fetus, 2DSTE-derived strain remains relatively stable and SR decreases from the second to the third trimester. Absolute SR values were relatively high compared with newborn values, which can be explained by the intrinsic properties of the heart and the expected changes in preload and afterload. Small, poorly compliant hearts with high heart rates will need fast wall shortening. Fetal cardiac growth throughout the pregnancy ensured that SV increased for any given wall shortening.
Table 13.2 presents all clinical studies reported to date in term newborn infants in the first days or weeks after birth. Most investigators reported on S L , but data on circumferential strain, radial strain (S R ), twist, or RV S L in healthy term infants were available from only one or two studies with only a few participants. Overall, the average S L reported in the studies was around −20%, ranging from −18% to −22%, and it remained relatively stable throughout the neonatal period.
| Study | Year | N | PNA (days) | Software | Frame Rate (/s) | S L (%) | SR L (1/s) | S C (%) | S R (%) | Twist (°) | RV S L (%) | RV SR L (1/s) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhang | 2010 | 29 | 15–28 | EchoPAC | >50 | 1.9 (1.2) | ||||||
| Elkiran | 2014 | 32 | 1–3 | XStrain | 50–75 | −11.2 (2.5) | −13.7 (2.9) | |||||
| Sehgal | 2013 | 20 | 0–2 | EchoPAC | >80 | −21.5 (2.7) | −1.76 (0.2) | |||||
| Schubert | 2013 | 30 | 7 | EchoPAC | 187 | −19.5 (2.1) | −2.37 (0.8) | −21.5 (4.2) | −2.56 (1.2) | |||
| Klitsie | 2013 | 28 | 1–3 | EchoPAC | −18.8 (3.5) | −19.7 (4.3) | 29.4 (12.2) | |||||
| Sehgal | 2014 | 21 | 3–4 | EchoPAC | >80 | −21.3 (2.8) | −1.75 (0.2) | |||||
| Jain | 2014 | 50 | 1–2 | EchoPAC | 80–100 | −21.2 (3.9) | ||||||
| Al-Biltagi | 2015 | 45 | 0–2 | EchoPAC | 65 | −19.0 (2.1) | 5.4 (2.4) | |||||
| Jain | 2017 | 50 | 0–1 | EchoPAC | 80–100 | −21.7 (1.9) | −2.05 (0.15) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree