SPECT and SPECT-CT Imaging Protocols
Daniela B. Husarik
Thomas F. Hany
Single-photon emission computed tomography–computed tomography (SPECT-CT) systems combine a SPECT gamma camera and a CT system. Depending on the system design, low- to high-quality CT scanners are used. The benefit of using such an integrated system is twofold, as in PET-CT: the transmission images can be used for transmission correction and also for anatomic coregistration when anatomic localization of a lesion is difficult. Unlike PET-CT systems, SPECT-CT systems can also be used in a planar mode. In fact, a planar nuclear medicine acquisition on the gamma camera combined with a CT scanogram permits fusion of planar images as well.
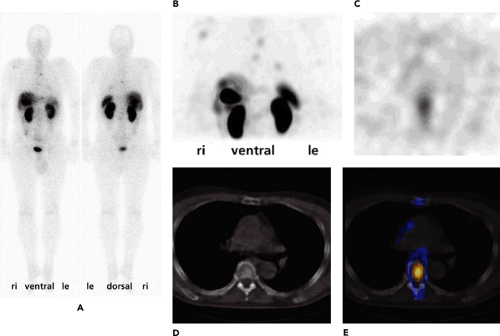
Clinically, the most relevant application of SPECT is myocardial perfusion imaging. There, SPECT-CT transmission measurements help to eliminate attenuation artifacts in the posterior cardiac wall. The usefulness of SPECT-CT, beyond transmission correction using transmission scanning, mainly occurs in tumor imaging based on radiopharmaceuticals such as iodine 131 (131I), iodine 123 [123I]MIBG, and indium 111 [111In]octreotide (Fig. 20.1); dual-peak parathyroid imaging; sentinel node scintigraphy in ENT and breast cancer; and lung perfusion studies with technetium 99m [99mTc]MAA. SPECT-CT is also used for skeletal imaging to pinpoint suggestive areas of uptake in the body by image coregistration. In these cases, attenuation correction is usually not needed. Instead of using an in-line SPECT-CT, a stand-alone CT system can be used, and images can be fused with dedicated computer software without difficulty, especially in bone scans. Overall, the use of SPECT-CT has significantly reduced the uncertainty of the location of radiotracer uptake in patients.
Introduction
Conventional nuclear medicine imaging has proven to be a functional imaging modality, but most nuclear images contain little anatomic information. CT, on the other hand, has demonstrated that it provides excellent anatomic information, but it contains little to no functional information. Image coregistration with various morphologic imaging modalities (CT or MRI) with dedicated software has substantial merit, as it results in significantly improved image interpretation, reducing the amount of uncertainty regarding the location of a lesion observed in a nuclear scan. The disadvantage of using software coregistration is that exactly coregistered patient positioning in both imaging modalities is difficult to guarantee. Extrinsic markers and mattresses providing “cast-like” forms increase the exactness of patient positioning during imaging with the two modalities intended for coregistration but are cumbersome logistically. In bone scans, this is not necessary, as there are sufficient anatomical landmarks on SPECT for later image fusion with coregistration software. Postprocessing to integrate the two data sets from different imaging devices can be time consuming. The breakthrough is achieved with integration of anatomic and functional imaging in one imaging device, a SPECT-CT gamma camera or a PET-CT camera (1,2).
SPECT-CT cameras have the advantage that all conventional nuclear medicine imaging can be performed with them, and when required, integrated anatomic and functional imaging can be done without the need for patient repositioning or rescanning elsewhere. No postprocessing is needed for image coregistration with such a system. Additionally or alternatively, when required, the CT data can be used for attenuation correction to improve SPECT image quality (3,4,5).
There is one key difference between SPECT-CT and PET-CT. The cost of the integrated device parts is heavily weighted toward CT if a high-end CT is used in a SPECT-CT system, and SPECT data acquisition is substantially slower than PET data acquisition. As a result, it may be much less attractive financially to own a SPECT-CT system with a high-end CT, as the expensive CT idles much of the time when the combined system is used for an examination, and billable examination costs for SPECT-CT are typically lower than for PET-CT.
SPECT-CT Hardware
Several systems are commercially available that integrate dual-head gamma cameras with different CT systems (e.g., the Siemens Symbia T, T2, and T6; the Philips Precedence 6 and 16 slice; and the GE Healthcare Infinia and Hawkeye). These systems have all-energy digital detectors with 5/8- or 1-inch special crystals, with an energy range from thallium to fluorodeoxyglucose (FDG). The axial scan length is 40 cm, with an effective field of view of 540 × 400 mm. In certain low-dose CT systems (like the Hawkeye system), the x-ray tube is mounted on the same slip-ring gantry as the two gamma camera heads. In these systems, the tube voltage is 140 kV, and a fixed current of 2.5 mA is used. The acquisition time for one slice is approximately 15 to 20 seconds, using data from a half scan to reconstruct one slice with a standard thickness of 10 mm and a maximum in-plane resolution of 256 × 256. CT data can be used for attenuation correction and anatomic information. No additional postprocessing is necessary for image coregistration. A typical acquisition time for a SPECT-CT on the Hawkeye with a 40-cm axial field of view is 40 to 60 minutes, depending on the type of radiopharmaceutical used and the field of view covered. In other systems, the CT system operates independently and is mounted in line with the center of the SPECT gantry, very much as in PET-CT systems. The CT systems used are medium to high-end and have up to 16-slice CT scanners. The major advantage of such systems is that, like PET-CT systems, they are able to acquire fully diagnostic, intravenous contrast–enhanced CT images in the region of interest.
As mentioned, SPECT in bone scans with technetium 99m [99mTc]phosphonates can be followed by a CT scan on a stand-alone machine. For bone scans, attenuation correction is not necessary, and images can be fused after completion of the examination with coregistration software.
Quality Assurance and Control
Quality control consists of daily, weekly, and trimonthly tests. On a daily basis, we always reset the gantry parameters and test the pressure-sensitive detector control for patient safety. Daily quality control for the CT simply involves performing a blank scan. A pulse height analysis acquisition, energy peak position, and energy resolution for the gamma camera are obtained by acquiring data from an extrinsic flood source and applying energy, linearity, and sensitivity corrections. The flood uniformity is calculated by using National Electrical Manufacturers Association (NEMA) standards. A sealed cobalt 57 (57Co) source is preferred to a 99mTc flood source because it is easier to handle and avoids possible contamination. This quality control test confirms the stability of the system performance characteristics.
Weekly quality control involves removing the collimators and performing an intrinsic flood source acquisition with a technetium point source with the recommended dose, distance, and height given by the manufacturer for a specific number of total counts per detector head at a given count rate per second. This weekly intrinsic quality control test calculates the pulse height analysis acquisition, energy peak position, and energy resolution for the gamma camera. A trimonthly center-of-rotation quality control test should also be performed.
Patient Scheduling
Optimization of patient throughput in a conventional nuclear medicine unit is highly dependent on the radiotracers used, the imaging protocols, proper patient preparation, and punctual arrival of patients for their examinations. The critical factor for throughput is the decision when to use SPECT-CT clinically. In systems where the CT is relatively slow, the prudent use of CT for attenuation correction for quantification or image fusion for localization of abnormal uptake will determine how many studies can be performed on the camera during the hours of operation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree