SPECT Radiopharmaceuticals for Perfusion Imaging and Tumor and Inflammation Localization
Gerrit Westera
In this chapter, the SPECT radiopharmaceuticals frequently used in clinical practice are discussed. Three major classes can be distinguished: perfusion, tumor, and inflammation markers. Perfusion radiopharmaceuticals are efficiently extracted from the blood upon passing the target organ, after which the radioactive label remains in the cells. Perfusion imaging with SPECT tracers is thus an organ-specific procedure. Tumor-seeking radiopharmaceuticals frequently accumulate in tumors rather nonspecifically because of increased metabolism and other physiologic processes due to rapid cell growth and proliferation. Sometimes the mechanisms are related to specific molecular structures on the tumor cells (receptors), and thus binding is more specific. The clinically most relevant tumor-seeking molecules are discussed. Infection and noninfectious inflammation are characterized by an increase in local capillary leakiness and the accumulation of white blood cells. Both these processes can be imaged by using radiolabels.
Introduction
SPECT imaging is widely available, and some very useful imaging agents have been developed since the inception of nuclear medicine. The most effective and most widespread agents are used in perfusion imaging of various organs or organ systems, tumor imaging, and imaging of inflammation. Although the perfusion imaging agents are typically used to image one organ, the tumor- and inflammation-seeking agents are intended in most instances to search foci of activity in the entire body. Some interesting agents exist that are directed against specific components of receptor systems, but few of these agents are in widespread use, and it is not clear whether they will find widespread introduction or are only precursors to similar “higher resolution” PET agents (see, e.g., Chapter 26).
Perfusion Imaging Agents
The ideal perfusion radiopharmaceutical is one that is completely extracted from the blood upon passing the target organ, after which the radioactive label remains in the
cells for a long time without being washed out so as to enable ample imaging time of a “quasi-steady-state tracer distribution” at a convenient time after injection. In practice, none of these conditions are fully met. A high extraction fraction with slow washout of the label afterwards is the normal situation. Perfusion imaging with SPECT tracers is an organ-specific procedure that requires different radiopharmaceuticals for various organs. Using radiopharmaceuticals for first-pass perfusion measurements has long ago lost attractiveness in view of the better organ-specific perfusion agents. Here, nuclear medicine has a clear advantage over other imaging techniques, where first-pass agents are the only ones that can be used for perfusion imaging, as dictated by toxicity considerations. The nuclear perfusion agents thus are described under organ system headings.
cells for a long time without being washed out so as to enable ample imaging time of a “quasi-steady-state tracer distribution” at a convenient time after injection. In practice, none of these conditions are fully met. A high extraction fraction with slow washout of the label afterwards is the normal situation. Perfusion imaging with SPECT tracers is an organ-specific procedure that requires different radiopharmaceuticals for various organs. Using radiopharmaceuticals for first-pass perfusion measurements has long ago lost attractiveness in view of the better organ-specific perfusion agents. Here, nuclear medicine has a clear advantage over other imaging techniques, where first-pass agents are the only ones that can be used for perfusion imaging, as dictated by toxicity considerations. The nuclear perfusion agents thus are described under organ system headings.
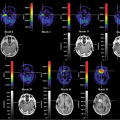
Brain Perfusion
Technetium 99m [99mTc]exametazime ([RR,SS]-4,8diaza-3,6,6,9-tetramethylundecane-2,10-dione bisoxime or HM-PAO) is an uncharged lipophilic and low molecular weight complex that easily crosses the blood–brain barrier. Approximately 3.5% to 7% passes into the brain by passive diffusion during the first minute after injection. After some initial washout during the first 2 minutes, the remainder of the activity is retained in the brain without loss for the next 24 hours. It is thought that the complex probably reacts with glutathione in the cell and thus becomes trapped (1).
During the labeling procedure, there is a risk of making a secondary [99mTc]exametazime complex that is hydrophilic and thus is not extracted quickly from the blood, causing higher background activity. This especially occurs in the presence of oxygen, and thus the labeling should be done with freshly eluted 99mTc pertechnetate, eluted from a generator that was last eluted within the previous 24 hours. If the eluate has to be diluted before the labeling procedure, it is helpful to dilute with saline that does not contain oxygen (e.g., stored under nitrogen).
Unless stabilized by chemical additives (cobalt [II] chloride), the labeled complex is not stable over longer periods of time and must be injected within 30 minutes after labeling; if stabilized, it must be injected within 5 hours. Quality control has to be performed before application to ascertain radiochemical purity.
[99mTc]bicisate (N,N′-[1,2-ethylenediyl]bis-L-cysteindiethylester dihydrochloride, 99mTc-ECD) is a stable complex 5% to 6.5% of which is taken up by the brain during the first few minutes (2,3).
The complex is slowly eliminated from the brain, but the regional distribution in the brain remains the same for at least 6 hours. The two-step labeling procedure should be done quickly with fresh, oxidant-free [99mTc] eluate in phosphate buffer, and quality control has to be performed before application to ascertain radiochemical purity. The preparation is stable for at least 8 hours.
Lung Perfusion
[99mTc] human albumin macroaggregates are particles with a diameter of 10 to 100 μm (4). Following injection into a superficial vein of the systemic venous circulation, the macroaggregates (6 × 104 to 7 × 105 particles in an adult) are carried to the first capillary filter (i.e., the capillary tree of the pulmonary artery system). The albumin macroaggregate particles do not penetrate the lung parenchyma (interstitial or alveolar) but remain in a temporary occlusive position in the lumen of the capillary. The technetium-labeled macroaggregates remain in the lungs for variable periods of time, depending on the structure, size, and number of particles. The larger aggregates have a longer biological half-life, whereas particles between 5 and 90 μm in diameter have a half-life ranging from 2 to 8 hours. This perfusion measurement is the paradigmatic wash-in perfusion experiment, as during the first pass 100% of the radiopharmaceutical is extracted.
The decrease in the pulmonary concentration is caused by the mechanical breakdown of the particles occluding the capillaries. The resulting recirculating albumin microcolloid is quickly removed by the macrophages of the reticuloendothelial system (essentially the liver and the spleen). The microcolloid is metabolized with the introduction of the radioactive label (99mTc) into the systemic circulation, from which it is removed and excreted in urine. The application of the radiopharmaceutical should be done within 8 hours after preparation.
Heart Perfusion
Monovalent, positively charged metals and metal complexes are taken up and retained by the myocardium, enabling the estimation of myocardial perfusion (5).
[201Tl]thallous chloride (T1/2 = 3.04 days) provides a cationic Tl+ solution. After intravenous injection of [201Tl]thallous chloride, the thallium rapidly leaves the blood, and approximately 90% is cleared after the first pass. The relative uptake depends on regional perfusion and on the cell extraction efficacy of different organs. The myocardial extraction fraction of 201Tl is about 85% during the first pass during stress, and the peak myocardial activity is 4% to 5% of the injected dose and relatively constant for about 20 to 25 minutes. The precise cellular uptake process is still questionable, but the sodium–potassium ATPase pump is probably involved, at least in part.
The muscular uptake is dependent on myocardial workload during the injection and compared with the resting condition, the uptake into myocardium and skeletal muscle is increased two- to threefold during exercise, with a consequent reduction of uptake into other organs.
[99mTc]sestamibi (99mTc[2-methoxyisobutylisonitrile]6, 99mTcMIBI) is a cationic complex that accumulates in vital myocardium in proportion to perfusion (1.2% of the injected dose [during rest] to 1.5% [during stress]). Under hypoxic conditions, extraction is diminished. The regional
myocardial distribution is comparable to that of 201Tl and remains stable from 0.5 (stress) or 1 (rest) up to at least 2 hours. Labeling must be done with oxidant-free 99mTc eluate (from a recently eluted generator) under heating. The radiochemical purity should be checked before application. The application should occur within 6 hours after labeling (6).
myocardial distribution is comparable to that of 201Tl and remains stable from 0.5 (stress) or 1 (rest) up to at least 2 hours. Labeling must be done with oxidant-free 99mTc eluate (from a recently eluted generator) under heating. The radiochemical purity should be checked before application. The application should occur within 6 hours after labeling (6).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree