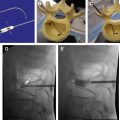
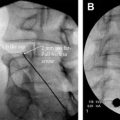
Spinal cord stimulation has been used successfully for more than 40 years. The application of an electrical impulse field on to the spinal cord is used with a battery generator source and a variety of either cylindrical or paddle/plate leads. Energy is delivered from either a conventional internal programmable generator or a rechargeable style battery. Many clinical conditions such as complex regional pain syndrome, failed back spinal syndrome, and extremity neuropathic pain involving the trunk and limbs are approved for its use. This device allows patients to live a successful life without pain.
Therapeutic electrical stimulation of the nervous system has developed enormously during the last 40 or so years, from the work that was performed by Shealy and colleagues to the point where tens of thousands of units have been implanted every year, and yet one of the biggest criticisms is that there is a lack of high-quality evidence of its efficacy.
Since the recent enlightenment regarding the neurophysiology of pain, it has been discovered that electrical stimulation of almost any part of the nervous system can have a dramatic useful purpose of modulating painful conditions, such as spinal conditions (failed back spinal syndrome [FBSS]), complex regional pain syndrome (CRPS) I and II, interstitial cystitis, gastroparesis, chronic pancreatitis, peripheral neuropathic pain, and transformed migraine headaches. It seems that therapeutic stimulation has the potential to continue to add to the knowledge of neurologic function; for example, what does relief of central pain afforded by motor cortex stimulation tell about the integration of motor and sensory systems in the brain? It seems that, at least to some extent, spinal cord stimulation (SCS) influences the intrinsic, already available, modulatory systems, whose function may or may not have been disturbed, to bring about normalization. This principle may apply to neuropathic pain and maladaptive changes occurring in ischemic syndromes, such as myocardial ischemia, peripheral vascular ischemia, and neuropathic ischemia.
The history of neurostimulation
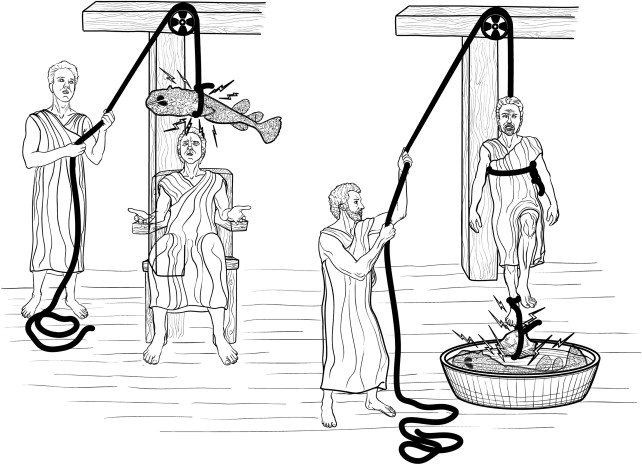
Electrical stimulation for the treatment of pain has been around, in one form or another, in every culture for thousands of years. It is said that, from circa 9000 bc , bracelets were used to prevent headaches and arthritis. Each culture acquired and/or discovered a type of fish that could, through electrical stimulation, stun or affect a person in one way or another. In the Nile Valley, Africa, electric catfish use electrical discharge to stun their prey. The ancient Egyptians acknowledged the power of the Nile catfish in tomb paintings. The ancient Greeks called the ray “Narke” or “numbness-producing,” from which the word “narcosis” was coined. The Romans called the ray “torpedo” from the word “torpor” because the name was synonymous with the effect. Conditions such as gout and headaches were treated by having the torpedo fish discharge its electrical charge close to the site of the painful condition ( Fig. 1 ).
The therapeutic application of electricity called “Franklinism” was named after the American statesman and scientist Benjamin Franklin, who, with his famous kite experiment in 1775, proved that lightening and electrostatic charge on a Leyden jar were identical. John Wesley, the founder of Methodism, extolled the virtues of electricity in his book, The Desideratum , and advocated electrotherapy for angina pectoris, gout, headaches, pleuritic pain, and sciatica.
Gate control theory and implantable stimulators
The theory by Melzack and Wall in 1965 postulated central inhibition of pain by nonpainful stimuli, a concept that had been predicted half a century ago by the English neurologist Sir Henry Head. In 1965, Wall recruited William Sweet, Head of Neurosurgery at Harvard Medical School, to clinically test the gate theory. Melzack and Wall proposed in their theory that nerve impulses from afferent fibers lead to spinal cord transmission (T) cells in the substantia gelatinosa. The firing of the projection neuron determines pain. The inhibitory interneuron decreases the chance that the projection neuron will fire. Firing of C fibers inhibits the inhibitory interneuron (indirectly), thereby increasing the chances that the projection neuron will fire. Firing of the large myelinated Aβ fibers activates the inhibitory interneuron, reducing the chances that the projection neuron will fire, even in the presence of a firing nociceptive fiber. The marriage of electricity to pain control in this paradigm of the gate control theory lies in the age-old principle of “counterirritation.” At first, they experimented on their own infraorbital nerves using needle-stimulating electrodes and on superficial nerves, such as the ulnar nerve, using superficial electrodes. Then they used transcutaneous or percutaneous stimulation in 3 patients who experienced partial or complete relief of pain during stimulation. Shealy (a neurosurgeon in La Crosse, Wisconsin) and colleagues thought that the “gate” could be best closed by stimulating the dorsal columns and confirmed this assumption experimentally in cats. In 1967, Shealy and his coworkers implanted a device ( Fig. 2 ) in a 50-year-old woman suffering from intractable carcinomatous pelvic pain and used a radiofrequency stimulator.
The circuit design was based on a modified Medtronic device (Medtronic, Inc, Minneapolis, MN, USA) for the stimulation of the carotid sinus to control angina and hypertension. The patient experienced approximately 50% relief from her pain, at times almost total control of her pain, and was extensively evaluated until Mortimer (from Shealy’s team) successfully defended his PhD thesis Pain suppression in man by dorsal column electroanalgesia in May 1968.
Gate control theory and implantable stimulators
The theory by Melzack and Wall in 1965 postulated central inhibition of pain by nonpainful stimuli, a concept that had been predicted half a century ago by the English neurologist Sir Henry Head. In 1965, Wall recruited William Sweet, Head of Neurosurgery at Harvard Medical School, to clinically test the gate theory. Melzack and Wall proposed in their theory that nerve impulses from afferent fibers lead to spinal cord transmission (T) cells in the substantia gelatinosa. The firing of the projection neuron determines pain. The inhibitory interneuron decreases the chance that the projection neuron will fire. Firing of C fibers inhibits the inhibitory interneuron (indirectly), thereby increasing the chances that the projection neuron will fire. Firing of the large myelinated Aβ fibers activates the inhibitory interneuron, reducing the chances that the projection neuron will fire, even in the presence of a firing nociceptive fiber. The marriage of electricity to pain control in this paradigm of the gate control theory lies in the age-old principle of “counterirritation.” At first, they experimented on their own infraorbital nerves using needle-stimulating electrodes and on superficial nerves, such as the ulnar nerve, using superficial electrodes. Then they used transcutaneous or percutaneous stimulation in 3 patients who experienced partial or complete relief of pain during stimulation. Shealy (a neurosurgeon in La Crosse, Wisconsin) and colleagues thought that the “gate” could be best closed by stimulating the dorsal columns and confirmed this assumption experimentally in cats. In 1967, Shealy and his coworkers implanted a device ( Fig. 2 ) in a 50-year-old woman suffering from intractable carcinomatous pelvic pain and used a radiofrequency stimulator.
The circuit design was based on a modified Medtronic device (Medtronic, Inc, Minneapolis, MN, USA) for the stimulation of the carotid sinus to control angina and hypertension. The patient experienced approximately 50% relief from her pain, at times almost total control of her pain, and was extensively evaluated until Mortimer (from Shealy’s team) successfully defended his PhD thesis Pain suppression in man by dorsal column electroanalgesia in May 1968.
Dorsal roots
Most dorsal root fibers, on entering the spinal cord, proceed toward the dorsal columns, where they bifurcate into ascending and descending branches. In comparison with longitudinal dorsal column fibers, dorsal root fibers have a curved shape, and they differ in orientation with respect to the spinal cord and the implanted electrodes. Dorsal root fibers average 15 μm in diameter. Proximal to the dorsal ganglion, the dorsal root fibers fan-out in an ascending, dorsomedial direction to form the rootlets that enter the spinal cord at different angles. Strujik and colleagues have studied the effect of the curvature of the dorsal roots on their electrical threshold.
Chronic back pain
Back pain is responsible for more than 80% of all back pain syndromes affecting Americans daily. One in every 14 individuals is affected by some kind of cervical, thoracic, or lumbar pain, meaning that out-of-work days can affect the work status of employers. Estimated annual cost for direct and indirect treatments is 20 to 60 billion dollars annually.
Most types of back pain are acute or subacute, resolving within a 6-week period of time normally. However, other estimates suggest that less than 30% of patients are completely improved within 3 months of treatment.
Chronic low back pain represents one of the most widespread and costly medical problems today; it is also a major cause of workplace absenteeism. Past analyses have demonstrated that more than 5 million people in the United States are afflicted with chronic low back pain. Conservative estimates place the annual cost of treatment at 25 billion dollars. A significant fraction of these dollars is attributable to more than 200,000 US patients yearly who elect for lumbosacral surgery to relieve their pain. Unfortunately, 20% to 40% of surgical patients experience persistent or recurrent pain.
Failed Back Spinal Syndrome
One important subset of patients includes those with the so-called failed back surgery syndrome. In the literature, this multidimensional syndrome has been used to describe various types of pain, including centrally located lumbosacral pain, buttock pain, gluteal pain, extremity pain, and diffuse lower back pain. Many published series emphasize the distinction between back pain and leg pain; however, details of the pain syndromes are usually lacking. The cause of these pains is very difficult to pinpoint. Some of the reasons are wrong level of surgery, psychological overlay, arachnoiditis, lumbosacral epidural fibrosis, vertebral microinstability, and recurrent disk herniations.
Although there are several paths as to why one develops a condition such as FBSS, the most common, unfortunately, is the poor selection for the surgery. This means that the patient may have had a psychological profile or physical pathology that was contraindicated or not appropriate for the surgical intervention. Furthermore, if the patient is misdiagnosed, the surgery is obviously incorrect and damaging. The most common misdiagnosis in these cases is arthritis misdiagnosed as lumbar disk disease. Often, improper selection and misdiagnosis follow from inadequate preoperative evaluation and diagnosis workup. A full diagnostic workup should include a medical and psychological evaluation. The medical evaluation should include a comprehensive physical examination and history, imaging, and relevant diagnostic procedures, such as radiography, computed tomography, magnetic resonance imaging, myelography, bone scanning, electromyography, discography, and various diagnostic injections, to help delineate the pain generator.
Surgery that is unnecessary may also be the cause of FBSS. Unnecessary surgery not only fails to treat the problem appropriately but also may worsen the patient’s condition. An unnecessary surgical excision of the nucleus pulposus from the normal disk is likely to increase the risk of chronic back pain by creating instability and malalignment. Needless surgery places patients at unnecessary risk for injured nerves, torn dura or arachnoid, cerebrospinal fluid (CSF) leakage, and later for possible wound infection or hemorrhage.
Complex Regional Pain Syndrome
CRPS, formerly recognized as reflex sympathetic dystrophy, is frequently misunderstood, misdiagnosed, and mistreated. First and foremost, reestablishing the function of the injured area is of utmost importance. Only 1 in 5 is able to return to work after having been diagnosed with the disease. It has been estimated to occur in approximately 1:2000 traumatic events. In 1994, the International Association for the Study of Pain proposed stringent diagnostic criteria and named the “complex regional pain syndrome type I.” Pain alleviation is secondary when it comes to the function of the injured area. As one of the author’s colleagues says frequently, “this is not a disease of the extremity; it is a disease of the nervous system.”
Initial work performed by the late Bonica provides an explanation of the disease-development stages. Stage 1, or the acute phase, is when the extremity is very sensitive to any form of touch, contact, or stimulus. The extremity may seem swollen, discolored, and stiff. Stage 2, or the dystrophic phase, is seen some 3 to 4 months after the initial injury. During this stage, the extremity begins to become contracted and remains swollen and very painful. The area now begins to feel cooler with respect to the other extremity. Unfortunately, during Stage 3, or the atrophic phase, the extremity becomes almost useless. If severe atrophy develops, uncal changes occur. Brittle nails form, and either excessive hair growth or sparse hair can result.
Physical therapy is mandatory to even hope for any improvement of the injured area. Functionality is the key. The injured area should respond to a combination of treatments. Medications should be started to improve sleep and inhibit neuropathic impulses from the injury; sympathetic inhibition is important to restore blood flow to the injury.
Conventional pain medication, physical therapy, sympathetic blocks, and transcutaneous electrostimulation of the nerves have all been used to help alleviate pain caused by the initial injury. SCS introduced by Shealy and colleagues in 1967 has been one of the most successful modalities used for alleviating pain, swelling, and stiffness today.
Of extreme importance in the clinical application of SCS to complex pain syndromes was that multiple arrays of electrodes with defined spacing would allow “capture” of pain (ie, the overlap of areas of paresthesia over the region of pain perception) better than a single array of electrodes. Law and Kirkpatrick showed that a defined area of the spinal cord, the physiologic midline, which differed from the anatomic midline, was crucial in the modulation of pain transmission. They also determined that medial dorsal column penetration was improved with bilaterally placed electrode arrays around the physiologic midline using staggered guarded cathodes with intercontact spacing of 4 mm and surface contact of 3 mm. (A guarded cathode is a selection of 3 adjacent electrodes with the midline electrode, the cathode [negative], having opposite polarity from the surrounding 2 anodal [positive] electrodes.) This would enhance the capture of pain in complex dynamic pain syndrome with axial back pain and CRPS. The concept of dual arrays of electrodes led the way to multichannel devices that allow the steering of paresthesia (ie, the moving of the active cathode in the longitudinal and transverse directions electronically) without the need for surgical intervention. A multichannel device ( Fig. 3 ) is one that allows for more than one lead to be active simultaneously, thus increasing the area of the spinal cord that can be covered.