Spleen, Pancreas, and Adrenal Glands
Ethan A. Smith
Jonathan R. Dillman
Sara O. Vargas
Peter J. Strouse
INTRODUCTION
Disorders of the spleen, pancreas, and adrenal glands are less common in children than are gastrointestinal, hepatobiliary, and genitourinary abnormalities, but nonetheless make up an important group of pediatric diagnoses. Radiologic imaging plays a critical role in evaluating a wide variety of splenic, pancreatic, and adrenal pathologies, including developmental, infectious, inflammatory, and neoplastic entities. In this chapter, the normal anatomy of the spleen, pancreas, and adrenal glands are discussed, followed by a description of common and selected rare pathologic processes that affect each of these organs.
SPLEEN
Imaging Techniques
Radiography
Radiography of the spleen is of limited value. Radiographs can be used to evaluate splenic size in the setting of suspected or known splenomegaly. The absence of a normal splenic shadow can be seen in children with sickle cell disease who have suffered autosplenectomy due to splenic infarction. Radiographs may detect splenic calcifications in sickle cell disease and granulomatous diseases, such as histoplasmosis and tuberculosis.
Ultrasound
Ultrasound is an excellent noninvasive, nonionizing modality for evaluation of the spleen. The spleen is usually readily imaged given its superficial position in the left upper quadrant of the abdomen. Sonography provides detailed information regarding splenic size, position, and vascularity, including assessment of the splenic artery and vein using Doppler sonography. The normal spleen is crescent shaped, demonstrates homogenous echotexture, and is usually slightly hyperechoic relative to the normal liver1 (Fig. 15.1). With higher frequency transducers, the spleen may have a somewhat granular or reticular appearance related to areas of red and white pulp that should not be confused with pathology.2
Both solid and cystic focal splenic lesions and their associated vascularity are also readily evaluated using ultrasound. Calcifications may be seen as hyperechoic foci with associated artifacts (twinkling artifact and/or posterior acoustic shadowing). Structures adjacent to the spleen also may be seen, for example, abnormal perisplenic portosystemic collateral vessels in the setting of portal hypertension.
Computed Tomography
Computed tomography (CT) is the most widely used imaging modality in the evaluation of pediatric splenic trauma. CT is also useful in assessment of the spleen in children with oncologic diseases and in suspected infectious splenic conditions. On noncontrast CT, the normal spleen demonstrates homogenous soft tissue attenuation. After intravenous administration of iodinated contrast material, the spleen typically demonstrates inhomogeneous enhancement on early (arterial) phase postcontrast images, with arc-like, striated, or focal areas of enhancement.3 This pattern of inhomogeneous enhancement is of unclear etiology, although some have attributed this appearance to differential rates of blood flow through the architecture of the spleen with alternating cords of red pulp and white pulp.3 On later postcontrast imaging phases, the normal spleen demonstrates homogeneous parenchymal enhancement.4
Magnetic Resonance Imaging
Similar to CT, MRI provides detailed evaluation of the spleen and splenic pathology. The normal spleen is homogeneously hyperintense on T2-weighted images and also demonstrates hyperintense signal on diffusion-weighted imaging. As with CT, on dynamic postcontrast images, the normal spleen demonstrates inhomogeneous enhancement on early phases and becomes more homogeneous on later phases3,4 (Fig. 15.2).
The superior tissue contrast of MRI related to the availability of various pulse sequences allows for detailed evaluation of focal splenic lesions, including solid masses, cystic lesions, and vascular abnormalities. Pulse sequences obtained both before and after the intravenous administration of gadolinium-containing contrast material generally are useful. In addition, MRI allows for characterization of diffuse splenic processes, such as splenic iron deposition in secondary hemochromatosis. Finally, dynamic contrast-enhanced MRI (DCE-MRI) excellently depicts the splenic arterial and venous vasculature, including aneurysms, thromboses, and perisplenic portosystemic collateral vessels in the setting of portal hypertension.
Nuclear Medicine
Several nuclear medicine techniques are available for imaging the spleen. Normal, functional splenic tissue can be identified using radiolabeled damaged red blood cells that are filtered and stored in the spleen. Radiolabeled sulfur colloid also localizes to the normal liver and spleen. In pediatric oncology patients, 18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET) imaging, often performed in conjunction with CT, has proven to be useful in evaluating the spleen for focal metastases as well as for diffuse involvement in lymphoma and other hematologic malignancies.5
Normal Anatomy
The spleen is the largest lymphatic organ in the human body and also functions as the primary hematopoietic organ during fetal development.6 The spleen contains two primary histologic elements: red pulp and white pulp. The red pulp of the spleen acts as a filter for damaged hematopoietic elements (mainly red blood cells) and also plays an important role in immunologic response.7 The white pulp serves as an important component of the lymphatic system.7 The spleen is derived from mesenchymal cells arising from the left side of the dorsal mesogastrum.6 The mesenchymal cells enlarge, develop vascularity, and subsequently fuse to become the trabeculae and capsule of the spleen, after which they are covered by a peritoneal lining derived from the coelomic epithelium.
Fusion of the individual mesenchymal elements is what gives rise to splenic clefts and the sometimes lobulated appearance of the spleen.6 The final position of the spleen in the left upper quadrant is determined by rotation of the stomach, with the spleen being posterior to the stomach and anterior to the tail of the pancreas.6 The spleen is attached to adjacent structures by the gastrosplenic ligament anteriorly, and the splenorenal and splenopancreatic ligaments medially.6
Fusion of the individual mesenchymal elements is what gives rise to splenic clefts and the sometimes lobulated appearance of the spleen.6 The final position of the spleen in the left upper quadrant is determined by rotation of the stomach, with the spleen being posterior to the stomach and anterior to the tail of the pancreas.6 The spleen is attached to adjacent structures by the gastrosplenic ligament anteriorly, and the splenorenal and splenopancreatic ligaments medially.6
The blood flow to the spleen is provided by the splenic artery, which is a branch of the celiac artery. The splenic artery bifurcates into segmental branches near the splenic hilum. The venous outflow from the spleen is provided by the splenic vein, which courses along the inferior and posterior aspect of the pancreas and then joins the superior mesenteric vein to form the main portal vein.
The spleen increases in size with age until puberty. Graphs and tables describing normal spleen sizes for both male and female pediatric patients as a function of age are available.8
Congenital Anomalies
Heterotaxy Syndromes (Asplenia and Polysplenia)
Asplenia and polysplenia are part of a complex association with visceral heterotaxy syndromes (i.e., situs ambiguous) and are associated with variable cardiac and other visceral congenital anomalies resulting from discordance of the normal left-right asymmetry of various organ systems, vascular structures, and cardiac chambers.7,9 The spleen is nearly universally involved in heterotaxy syndromes.
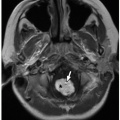
Imaging findings vary depending on the type of abnormality present. The spleen may be absent (asplenia), multiple (polysplenia), or unusually lobulated, or there may be single spleen located in the right upper quadrant.10 In polysplenia, the multiple spleens are often located in the right upper quadrant10 (Fig 15.3). Congenital heart disease is common in patients with heterotaxy syndromes. Importantly, in pediatric patients with heterotaxy with polysplenia, the incidence of congenital heart disease is ˜50% to 90%, whereas in patients with asplenia, the incidence
of congenital heart disease is 99% to 100%.11 Congenital heart disease in asplenia is often more complex and is commonly associated with anomalous pulmonary venous return. Additional congenital anomalies associated with heterotaxy with polysplenia include interruption of the inferior vena cava, a truncated pancreas, and a preduodenal location of the portal vein.10,12
of congenital heart disease is 99% to 100%.11 Congenital heart disease in asplenia is often more complex and is commonly associated with anomalous pulmonary venous return. Additional congenital anomalies associated with heterotaxy with polysplenia include interruption of the inferior vena cava, a truncated pancreas, and a preduodenal location of the portal vein.10,12
Accessory Spleen
Accessory or supernumerary spleens, sometimes called splenules, are a common normal variant, occurring in between 10% and 30% of the population.6,9,13,14 Accessory spleens are usually located near the splenic hilum, but can also be found along the supporting splenic ligaments, along the splenic vessels, or even within the pancreas (most often the tail) or in the wall of the stomach.9.
On imaging, accessory spleens are often solitary, but can be multiple. Accessory spleens vary in size, but are usually around 1 cm in diameter.9 They are usually smooth and rounded or oval in shape and have identical echogenicity, CT attenuation, and MR signal characteristics to the spleen itself.13 Accessory spleens are typically of no clinical significance, except in certain uncommon instances. For example, in hereditary spherocytosis, splenectomy may be ineffective if an accessory spleen is left behind at surgery.14 Intrapancreatic accessory spleens can cause diagnostic confusion as the intrapancreatic splenic tissue can be confused with a pancreatic neoplasm
Wandering Spleen
Failure of development of the normal gastrosplenic and splenorenal ligaments, which fix the spleen in its normal anatomic position in the left upper quadrant, can result in an abnormally elongated splenic attachment.9,13,14 The end result is a hypermobile spleen that can change position within the abdomen and may be confused with a mass. Wandering spleen is most common in children and young women.9 Clinically, wandering spleen may be an incidental finding, although in some cases this entity can be symptomatic because of chronic or intermittent torsion of the spleen resulting in ischemia and occasionally infarction. Gastric outlet obstruction due to gastric volvulus or mass effect on the stomach has also been described.15
Plain radiography may demonstrate the absence of a normal splenic opacity in the left upper quadrant. Ultrasound, CT, and MRI can demonstrate otherwise normal spleen in an ectopic location, with a lack of spleen in the normal anatomic position (Fig 15.4).
In cases of wandering spleen with torsion, patients present clinically with either acute or chronic abdominal pain.16 Imaging findings suggestive of torsion include twisting or swirling of the splenic vessels, lack of enhancement or Doppler flow in the spleen in cases of infarction, and adjacent reactive inflammatory changes.9 Treatment consists of either surgical fixation of the spleen (splenopexy) or splenectomy.16
Congenital Splenic Cyst
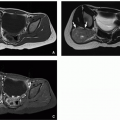
Also known as epithelial cysts, congenital splenic cysts are relatively uncommon. Differentiation of congenital splenic cysts from the more common splenic pseudocysts is not possible on imaging. Only upon histologic examination can the distinction be made by demonstrating an epithelial lining in the congenital splenic or epithelial cyst (Fig. 15.5).
Congenital splenic cysts demonstrate typical imaging features of simple cysts. On ultrasound, they are well-defined and homogeneously anechoic, whereas, at CT, they measure fluid attenuation. On MRI, congenital splenic cysts are homogeneously low in signal on T1-weighted sequences and high in signal on T2-weighted sequences (Fig. 15.6). Occasionally, these lesions may contain thin septations or debris.1 Cysts should not enhance after the administration of intravenous contrast material. Clinically, congenital splenic cysts are most often of no clinical significance and generally do not require treatment or resection. The differential diagnosis for a cystic lesion within the spleen includes splenic pseudocysts (most often posttraumatic), infectious cystic lesions, and lymphatic malformations.4
Infectious and Inflammatory Disorders
Fungal Infection
Fungal infections in the spleen are encountered almost exclusively in immunocompromised children.17 Candida are the most common species, followed by Aspergillus and Cryptococcus.18,19 Clinical presentation is usually nonspecific, with variable signs and symptoms of infection depending on the patient’s immune status. As opposed to bacterial abscesses, which are often larger and solitary, fungal infection commonly demonstrates multiple small (1 cm or less) splenic lesions, often termed microabscesses.18,20
On ultrasound, fungal microabscesses are typically hypoechoic and may have a target or “bull’s-eye” appearance17
(Fig. 15.7). They may be difficult to visualize on noncontrast CT, but after intravenous contrast material administration, the lesions are typically multiple and low in attenuation relative to the spleen.19 MRI may be the most sensitive method for detecting small microabscesses.20 The lesions may be inconspicuous on precontrast T1-weighted sequences, but are typically hyperintense on T2-weighted sequences and enhance less than the adjacent spleen after intravenous gadolinium-based contrast material administration.20 Affected pediatric patients commonly have hepatic lesions as well.
(Fig. 15.7). They may be difficult to visualize on noncontrast CT, but after intravenous contrast material administration, the lesions are typically multiple and low in attenuation relative to the spleen.19 MRI may be the most sensitive method for detecting small microabscesses.20 The lesions may be inconspicuous on precontrast T1-weighted sequences, but are typically hyperintense on T2-weighted sequences and enhance less than the adjacent spleen after intravenous gadolinium-based contrast material administration.20 Affected pediatric patients commonly have hepatic lesions as well.
Splenic calcifications may be a manifestation of subacute or remote infection, including prior infection with the endemic fungal pathogen Histoplasma capsulatum. Histoplasmosis is the most common systemic fungal infection in the United States.21 Blastomyces dermatitidis and Coccidioides sp. are the other common endemic fungal pathogens found in the United States.22 Acute splenic involvement is rare in the immunocompetent child but can present as splenic enlargement and multiple hypoechoic or low-attenuation splenic lesions, accompanied by systemic symptoms (Fig 15.8). Calcifications are the most common manifestation of remote infection and are usually punctate and multiple (Fig. 15.9). Occasionally, calcifications can be identified on abdominal radiography, but they are best demonstrated as hyperechoic foci on ultrasound, or round, punctate high-attenuation foci on CT.
Bacterial Infection
The most frequent manifestation of bacterial infection in the spleen is a pyogenic abscess. The majority of splenic abscesses are due to hematogenous spread of infection. Other, less common etiologies include penetrating trauma, direct extension from adjacent organs, or superinfection after splenic infarction.18,19 Clinically, affected pediatric patient with signs of infection, including fevers and abdominal pain, usually localized to the left upper quadrant.
Plain radiographs are not useful in evaluation of a suspected splenic abscess. Sonography is the diagnostic test of choice in children, given the lack of ionizing radiation and noninvasive nature. Ultrasound typically shows a hypoechoic or anechoic focal lesion, depending on the complexity of internal contents. The lesion may have mural irregularity or septations. Doppler evaluation often shows adjacent hyperemia due to an
inflammatory response. If needed, CT or MRI can be performed and demonstrate a complex cystic lesion, appearing relatively low in attenuation on CT and hyperintense in T2-weighted signal on MRI. After intravenous contrast material administration, there is usually a thick or irregular enhancing rim, but no central enhancement.19 Foci of gas may be evident, most readily demonstrated on CT.18 On MRI, diffusion-weighted imaging may be helpful by demonstrating restricted diffusion on high b-value images, indicating an abscess.
inflammatory response. If needed, CT or MRI can be performed and demonstrate a complex cystic lesion, appearing relatively low in attenuation on CT and hyperintense in T2-weighted signal on MRI. After intravenous contrast material administration, there is usually a thick or irregular enhancing rim, but no central enhancement.19 Foci of gas may be evident, most readily demonstrated on CT.18 On MRI, diffusion-weighted imaging may be helpful by demonstrating restricted diffusion on high b-value images, indicating an abscess.
Treatment is with antibiotic therapy, with or without percutaneous image-guided drainage. Rarely, surgical splenectomy may be required.18
Another relatively common bacterial infection that can rarely affect the spleen is Bartonella sp., which is the pathogen responsible for “cat-scratch disease.” Patients present clinically with fever and lymph node enlargement, and occasionally the spleen may become enlarged or may demonstrate multiple small microabscesses, which appear hypoechoic on ultrasound, low attenuation on CT, and hyperintense on T2-weighted MRI sequences.18
Mycobacterium
Mycobacterium species, most commonly M. avium-intracellulare and M. tuberculosis, can also affect the spleen. Similar to fungal infections, acute involvement of the spleen is rare in the immunocompetent host, but can be seen in immunocompromised children with disseminated disease, manifesting on imaging as either nonspecific splenic enlargement or multiple small splenic lesions. Adjacent lymph nodes may also be enlarged, and they may appear relatively low in attenuation on CT.18
Echinococcal Infection
Infection with Echinococcus sp. is rare in the developed world but continues to be a common source of disease worldwide.23 Dogs and similar carnivorous species are the definitive hosts. Humans are secondarily infected by ingesting water that is contaminated by feces containing the parasite. Once ingested, the parasite is absorbed through the duodenal mucosa and passes into the portal vein. Most echinococcal infections involve the liver, but spread to other organ systems also occurs, often secondary to rupture of hepatic lesion with secondary dissemination.18 Splenic involvement is relatively rare, with a reported prevalence of between 0.9% and 8% of cases.23
The most common appearance on imaging is that of a solitary cystic lesion which may or may not demonstrate mural calcification. Occasionally separation of the cyst layers may result in a visible floating membrane within the cyst. Smaller “daughter cysts” may also be present adjacent to the primary lesion.18,23
TABLE 15.1 Splenic Neoplasms in Children | ||||||||
|---|---|---|---|---|---|---|---|---|
| ||||||||
Neoplastic Disorders
Splenic neoplasms, both benign and malignant, are rare in children, and they can be categorized by their site of origin into hematolymphoid tumors, nonhematolymphoid tumors, and tumor-like lesions.24 Lymphoid tumors arise from the white pulp and are the most common type of splenic neoplasm. Vascular neoplasms, such as angiosarcoma, arise from the red pulp and are less common24 (Table 15.1).
Benign Neoplasms
Hamartoma
Splenic hamartomas are rare, benign lesions consisting of a proliferation of elements resembling red pulp. Hamartomas occur at all ages and do not demonstrate a gender predilection.24 There is an association between splenic hamartomas and genetic conditions that predispose to other hamartomatous lesions, most commonly tuberous sclerosis.24 Most splenic hamartomas are asymptomatic and discovered incidentally. Occasionally, large hamartomas may be palpable on physical examination and cause mass effect. Pathologically, splenic hamartomas are well-circumscribed nodules characterized histologically by disorganized red pulp, often in a fibrotic background, without intervening malpighian corpuscles (Fig. 15.10).
Ultrasound is usually the primary imaging modality for assessing suspected or known splenic hamartomas. These lesions typically appear as circumscribed, usually round masses, which are typically with homogeneously isoechoic or hyperechoic relative to the adjacent normal spleen.24 Occasionally lesions may be heterogenous, have cystic areas, or even have calcifications.24 On Doppler ultrasound, hamartomas often demonstrate increased blood flow relative to adjacent spleen.25 Splenic hamartomas may be difficult to identify on CT, as the lesions are generally isoattenuating or slightly hypoattenuating to adjacent normal spleen on both noncontrast and postcontrast images. The only clue to the presence of a splenic hamartoma may be focal distortion of the contour of the spleen. At MRI, these lesions may be similar in signal intensity to normal spleen on T1-weighted images, but can be heterogeneously isotense or slightly hyperintense on T2-weighted images4,24 (Fig. 15.11).
Malignant Neoplasms
Angiosarcoma
Angiosarcoma is a rare, aggressive malignant neoplasm that can arise within the spleen. This tumor is most common in adults, with most cases presenting in the sixth and seventh decades of life; however, cases have been reported in pediatric patients, including young children.26,27 Affected pediatric patients typically present with nonspecific left upper quadrant pain and splenomegaly. Metastases are often present at the time of diagnosis.28 Treatment is focused on splenectomy to remove the tumor and postoperative chemotherapy. The prognosis is generally poor, with only 20% of patients surviving >6 months after diagnosis.26
Ultrasound, CT, and MRI all demonstrate an aggressive splenic mass, usually with marked enlargement of the spleen.28 On ultrasound, the mass is usually heterogeneous, with areas of cystic change reflecting necrosis and
internal hemorrhage.24 CT may demonstrate multiple heterogeneous and hypervascular masses replacing the spleen. Areas of hemorrhage and calcification may also be present.24,28 At MRI, these lesions are often heterogeneous on both T1-weighted and T2-weighted sequences with heterogeneous enhancement after intravenous contrast material administration.28
internal hemorrhage.24 CT may demonstrate multiple heterogeneous and hypervascular masses replacing the spleen. Areas of hemorrhage and calcification may also be present.24,28 At MRI, these lesions are often heterogeneous on both T1-weighted and T2-weighted sequences with heterogeneous enhancement after intravenous contrast material administration.28
Lymphoma
The spleen is the largest lymphatic organ in the body, and, as such, it is commonly involved in both Hodgkin and non-Hodgkin lymphoma. Splenic involvement is present in up to 35% of children with Hodgkin lymphoma at the time of diagnosis.29 Accordingly, surgical splenectomy with pathologic evaluation of the spleen used to be a standard part of the initial diagnostic staging.30 The spleen is less commonly involved in non-Hodgkin lymphoma, but certain subtypes frequently involve the spleen.29 Current imaging techniques have obviated the need for surgical staging of the abdomen, but defining the extent of disease within the abdomen remains a critical part of radiologic disease evaluation at diagnosis and follow-up.
Radiologically, four different patterns of splenic involvement by lymphoma have been described, including diffuse infiltration, tiny nodules (miliary pattern), multiple larger nodules, and solid bulky masses28 (Fig. 15.12). Diffuse infiltration may only be apparent as splenomegaly at imaging. Discrete lymphomatous masses are generally hypoechoic on ultrasound and low attenuation relative to the normal spleen on contrast-enhanced CT,28 but both ultrasound and CT have been shown to have suboptimal sensitivity. As such, both 18F-FDG-PET/CT and MRI have emerged as important imaging tools to evaluate for splenic lymphoma. The sensitivity and specificity of 18F-FDG-PET/CT are excellent, with lymphomatous involvement appearing as foci of increased metabolic activity, although the ionizing radiation used for imaging is a drawback, especially in pediatric patients. At MRI, T2-weighted sequences alone have been shown to have high specificity, but relatively low sensitivity. Diffusion-weighted imaging can be helpful but also can be
limited because the normal spleen is relatively high in signal intensity making hyperintense lymphomatous deposits less conspicuous.31 However, the addition of DCE-MRI has shown promise in delineating splenic involvement.31 During DCE-MRI, lymphomatous deposits are seen as areas of focal signal hypointensity on the earlier phases of contrast enhancement.31
limited because the normal spleen is relatively high in signal intensity making hyperintense lymphomatous deposits less conspicuous.31 However, the addition of DCE-MRI has shown promise in delineating splenic involvement.31 During DCE-MRI, lymphomatous deposits are seen as areas of focal signal hypointensity on the earlier phases of contrast enhancement.31
Leukemia
The most common splenic manifestation of pediatric leukemia is diffuse enlargement of the spleen. Other relatively common splenic manifestations in leukemia are that of infection due to immunocompromise, as described previously. There are reports of pathologic splenic rupture as a presenting manifestation of leukemia or as a complication in patients with known leukemia.32
Metastases
Splenic metastases are relatively rare, occurring in 2% to 9% of all cancer patients, and are much less common in children than adults.28,33 Tumors that metastasize to the spleen tend to be the more aggressive malignancies, such as sarcomas or advanced neoplasms with widespread metastatic disease. In general, splenic metastases present as focal splenic lesions, generally hypoechoic on ultrasound and low attenuation on contrast-enhanced CT. At MRI, lesions are usually isoto hypointense on T1-weighted images and variable signal intensity on T2-weighted images and demonstrate relative hypoenhancement after intravenous gadolinium-based contrast material administration28 (Fig. 15.13). Additional sites of metastatic disease within the abdomen are often present as well.
Traumatic Disorders
In blunt trauma, the spleen is the most commonly injured abdominal solid organ in both children and adults.34,35 The location of the spleen in the left upper quadrant as well as its highly vascular nature predispose the organ to injury during blunt thoracoabdominal trauma. Contrast-enhanced CT is the primary imaging modality used in suspected splenic trauma.34,36 Occasionally ultrasound may be considered, but has been shown to have relatively low sensitivity in children.36,37 The use of contrast-enhanced ultrasound for evaluation of pediatric splenic trauma has been reported, but this technique is not yet widely available for use in the United States.38 Nuclear medicine studies and MRI do not generally play a role in the workup of suspected pediatric splenic trauma. Angiography may be utilized for treatment but is generally no longer used for diagnostic purposes.
Types of Splenic Injuries
Specific types of splenic injuries in blunt trauma include subcapsular and perisplenic hematomas, lacerations, contusions, infarctions, and vascular injuries. Although most splenic injuries have accompanying free intra-abdominal fluid (often blood) or hematoma, up to 25% of pediatric splenic trauma patients do not have fluid or blood elsewhere in their abdomen.39
A subcapsular hematoma implies extraparenchymal hemorrhage that is confined within the capsule of the spleen. At CT, a subcapsular hematoma appears as a lenticular or cres-centic collection of blood along the periphery of the spleen with mass effect upon the adjacent parenchyma.34 The blood products can be variable in attenuation, depending on the timing of imaging relative to the trauma, but are usually higher in attenuation than simple fluid (35 to 80 HU) in the setting of acute trauma. Extracapsular, perisplenic hematoma
implies blood products adjacent to the spleen and indicates that the splenic capsule has been disrupted.
implies blood products adjacent to the spleen and indicates that the splenic capsule has been disrupted.
A splenic laceration appears as discrete linear, branching, or stellate low-attenuation area extending through the splenic parenchyma34 (Fig. 15.14). If there is active bleeding, there may be high-attenuation iodinated contrast material pooling in or near the laceration, implying active extravasation of blood and contrast material. Lacerations can disrupt the vascular supply to the spleen resulting in splenic infarction (devascularization). Infarcted areas of spleen typically appear as geographic areas of low attenuation on contrast-enhanced CT (Fig. 15.15).
It is important to look for other associated injuries as well. In adults, up to 35% of patients with splenic injuries have other intra-abdominal injuries apparent on CT, and up to 80% have extra-abdominal injuries, including rib fractures and body wall injuries.34
Grading Splenic Injuries
Grading splenic injuries allows clinicians caring for pediatric trauma patients to have a standardized reference for injury severity and is increasingly being used to guide nonoperative management in pediatric splenic blunt trauma.34 A commonly used grading system for the severity of splenic trauma was created by the American Association for the Surgery of Trauma (AAST), which takes into account the types of injuries, number of injuries, depth of specific injuries, and any associated infarction, and results in a severity grade of 1-5 (1 = least severe; 5 = most severe).40
Complications from Splenic Injuries
Nonoperative management is now the standard of care for pediatric patients with blunt splenic trauma who are hemodynamically stable.41 Although rare, a few patients may develop delayed complications, including vascular injury with pseudoaneurysm formation, infection of posttraumatic fluid collections, and pseudocyst formation.
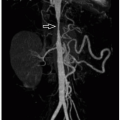
Pseudoaneurysms, or contained vascular rupture, occur as the result of arterial wall injury. Splenic artery pseudoaneurysms have been reported to occur in up to 5% of pediatric splenic injuries.42 Although rare, these lesions are clinically significant as there is a small risk of rupture with resultant life-threatening hemorrhage. On ultrasound, a splenic artery pseudoaneursym typically appears as a rounded hypo- or anechoic cystic structure, often located near the splenic hilum. With color Doppler ultrasound, there can be variable amounts of internal blood flow depending on any associated thrombus. Doppler ultrasound sometimes may reveal an alternating pattern of color signal, sometimes referred to as a “yin-yang” sign. Spectral Doppler ultrasound sampling of the neck of the pseudoaneurysm can confirm the diagnosis
by demonstrating to-and-fro blood flow going into and coming out of the pseudoaneurysm. On CT and MRI, the lesion appears as an avidly enhancing structure that should be similar in attenuation or signal intensity to adjacent enhanced vascular structures.
by demonstrating to-and-fro blood flow going into and coming out of the pseudoaneurysm. On CT and MRI, the lesion appears as an avidly enhancing structure that should be similar in attenuation or signal intensity to adjacent enhanced vascular structures.
Perisplenic and intrasplenic fluid collections are relatively common after blunt splenic trauma due to liquefaction and degradation of blood products. Occasionally, these collections can become infected and form abscesses with similar imaging features to abscesses elsewhere in the body, including an irregular enhancing wall, internal locules of gas, and adjacent inflammatory changes. As mentioned previously, splenic pseudocysts can be the result of trauma. These lesions lack an epithelial lining, differentiating them from epithelial or congenital splenic cysts. Imaging features are indistinguishable from other benign cystic lesions at ultrasound, CT, and MRI. Occasionally, large pseudocysts may require percutaneous drainage.4
Vascular Malformations
Vascular malformations can occur within the spleen and may be either isolated or associated with an underlying syndrome, such as Klippel-Trenaunay syndrome43 (Fig. 15.16). Imaging features of splenic vascular malformations are similar to those elsewhere in the body. On ultrasound, slow-flow vascular malformations are usually well-defined, anechoic, or hypoechoic lesions that may have lobulations or septations. Internal Doppler blood flow is variable depending on whether the lesion is predominantly venous or lymphatic. On CT, postcontrast enhancement of the lesion depends on the exact histologic nature of the lesions (venous versus lymphatic) as well as the phase of contrast enhancement.
MRI is the imaging modality of choice for characterizing splenic vascular malformations. The lesions are usually predominantly hyperintense on T2-weighted images with variable signal intensity on T1-weighted images (Fig. 15.17). In venous malformations, phleboliths may be identified as rounded T2-weighted hypointense filling defects, or rounded hypointense structures on gradient-recalled echo sequences. Predominantly venous malformations show enhancement, especially on more delayed postcontrast images. Predominantly lymphatic malformations generally only show thin, uniform enhancement of the walls and any internal septations. If there has been prior hemorrhage or infection, thin mural calcifications may be present, most easily seen on CT but also potentially visible on plain radiographs, ultrasound, and MRI.24
Splenomegaly
The spleen normally increases in size throughout childhood, becoming larger as the child grows. There are published normal values for pediatric splenic length based on age and gender.8 Splenomegaly is often defined as enlargement of the spleen >2 standard deviations above the mean expected length for patient age and gender (Fig. 15.18). The causes of splenomegaly are multiple, including portal hypertension (e.g., due to cirrhosis or chronic portal vein occlusion), infiltrative splenic disorders, neoplastic conditions, hyperfunction of the spleen (i.e., hypersplenism), infections (e.g., mononucleosis), and congenital disorders.4
Hemoglobinopathies
The red pulp of the spleen acts as a filter for damaged or abnormal red blood cells, to remove them from circulation and begin to recycle their components into new red blood cells. As such, several congenital conditions that affect red blood cells can affect the spleen. Examples of these conditions include congenital hemoglobinopathies, such as sickle cell disease and hereditary spherocytosis.
Sickle Cell Disease
Sickle cell disease is an autosomal recessive condition that results in abnormal hemoglobin molecules (HbS) that are prone to polymerize when in their deoxygenated state. This polymerization results in morphologically abnormal red blood cells, limiting the erythrocytes’ ability to deform and resulting in vascular occlusions.44 Sickle cell disease affects the spleen in two discrete ways. First, the spleen has to function to remove the abnormal red blood cells, which accumulate and fill the red pulp. In younger patients, this relative hyperfunction of the spleen can result in anemia and thrombocytopenia, a syndrome termed “splenic sequestration.”44 Second, the spleen, as an end-organ, has little or no collateral blood flow, making it vulnerable to vascular occlusions and infarction.
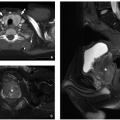
Imaging manifestations of splenic involvement with sickle cell disease are variable. In acute splenic sequestration, the spleen is diffusely enlarged. Variable findings of splenic infarction can occur over time. Infarcted areas generally appear as wedge-shaped or geographic areas of capsular-based hypoenhancement on CT or MRI.4 Occasionally, preserved islands of normal spleen can simulate splenic masses45 (Figs. 15.19 and 15.20). The end result of splenic infarction is a small calcified spleen, or in cases of complete infarction, the spleen may be nonvisualized.
Patients with hemoglobinopathies often undergo multiple blood transfusions. The iron associated with multiple blood transfusions can be deposited in the reticuloendothelial system and results in secondary hemochromatosis. Imaging manifestations of iron deposition are best evaluated using MRI, based on the principle that sequences with longer echo times (TE) result in susceptibility artifact and greater loss of signal than is normal. The amount of parenchymal iron can be quantified in the form of a T2* (or R2*) value. The most
commonly affected organs in secondary hemochromatosis include the liver, spleen, pancreas and bone marrow.
commonly affected organs in secondary hemochromatosis include the liver, spleen, pancreas and bone marrow.
Hereditary Spherocytosis
Hereditary spherocytosis is a common heritable disorder of red blood cells in which the erythrocytes have an abnormal spherical shape with resultant decreased surface area and lack of the ability of the cell to deform, all due to abnormal surface protein expression and disruption of normal phospholipid chains in the cell membrane.46 Affected pediatric patients present clinically with variable degrees of anemia and splenomegaly as a result of sequestration of the abnormal erythrocytes within the spleen with subsequent phagocytosis of abnormal erythrocyte membranes by splenic macrophages.
The most common imaging finding in hereditary spherocytosis is splenomegaly, which can range from mild to severe.
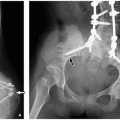
Children may also have gallstones as a result of increased red blood cell turnover47 (Fig. 15.21).
Children may also have gallstones as a result of increased red blood cell turnover47 (Fig. 15.21).
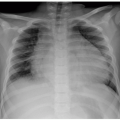
 FIGURE 15.21 Hereditary spherocytosis. Supine abdominal radiograph is a 16-year-old boy with hereditary spherocytosis shows splenomegaly (asterisk) and calcified gallstones (arrow). |
Treatment is usually supportive, with close monitoring of anemia and transfusions when necessary, although, in some cases, splenectomy may be performed to prevent red blood cell sequestration.46 Occasionally, partial splenectomy or partial splenic embolization may be performed to reduce the future risk of infectious complications related to asplenia. Splenectomy specimens are usually large and show an expanded red pulp (Fig. 15.22).
PANCREAS
Imaging Techniques
Radiography
The normal pancreas is not visible on plain radiographs. Occasionally, radiography may demonstrate pancreatic calcifications in chronic pancreatitis, or mass effect in the setting of a large pancreatic mass or pseudocyst.
Ultrasound
Portions of the pancreas may be difficult to visualize with sonography because of its retroperitoneal location and artifacts from overlying gas-filled stomach and loops of bowel. However, the pancreatic head and uncinate process are usually readily visualized adjacent to the duodenum. With appropriate graded compression, the entire pancreas often can be visualized, especially in younger children. Pancreatic parenchymal echogenicity, focal lesions, and ductal dilation can all be assessed by ultrasound.
Computed Tomography
Contrast-enhanced CT is the primary imaging modality in the setting of suspected pancreatic trauma and in acute conditions of the pancreas, such as acute necrotizing pancreatitis. CT can also readily demonstrate pancreatic atrophy, parenchymal calcifications, and ductal dilation in the setting of chronic pancreatitis or ductal obstruction. In the setting of suspected pancreatic masses, CT allows assessment of the size of the mass, its relationship to adjacent structures, and any evidence of local or distant abdominal metastatic spread. Detailed anatomic assessment of the pancreatic duct can be difficult given the limited contrast resolution of CT compared to MRI.
Magnetic Resonance Imaging
MRI, with its excellent contrast resolution, provides a detailed assessment of both the pancreatic parenchyma and the pancreatic ductal system. T2-weighted sequences, especially when fat saturation is applied, readily depict pancreatic inflammation, peripancreatic edema/fluid, and a variety of cystic lesions. Multiphase postcontrast imaging after intravenous administration of gadolinium-based contrast material can evaluate enhancement of the pancreatic parenchyma as well as characterize focal pancreatic lesions.
Magnetic resonance cholangiopancreatography (MRCP) can be used to evaluate the anatomy of the pancreatic ductal system. MRCP utilizes heavily T2-weighted pulse sequences in order to take advantage of the intrinsically long T2 relaxation times of fluid-containing structures, including bile ducts and pancreatic ducts.48 A variety of 2D and 3D MRCP techniques exist; however, 3D techniques are most commonly employed in modern MRCP protocols. When specifically
imaging the pancreatic duct, some have advocated the use of secretin, a hormone that causes increased production and secretion of pancreatic fluid, in order to dilate the duct and make the anatomy more evident; however, recently the utility of this technique has been called into question.49
imaging the pancreatic duct, some have advocated the use of secretin, a hormone that causes increased production and secretion of pancreatic fluid, in order to dilate the duct and make the anatomy more evident; however, recently the utility of this technique has been called into question.49
Nuclear Medicine
Nuclear medicine imaging plays a limited role in evaluation of pediatric pancreatic abnormalities. In patients with suspected neuroendocrine tumors, somatostatin receptor (octreotide) scintigraphy may play a role in characterizing the tumor and in the evaluation for metastatic disease. In malignant tumors of the pancreas, 18F-FDG-PET/CT may play a role in disease staging and surveillance.
Normal Anatomy
The pancreas has both endocrine and exocrine functions, with its primary endocrine role in control of glucose homeostasis and its primary exocrine function being to assist in the digestion of food.50 Pancreatic endocrine function is carried out by five distinct cell types organized into functional units termed “islet of Langerhans.”50 These five cell types include β-cells, which produce insulin; α-cells, which produce glucagon; δ-cells, which produce somatostatin; pancreatic polypeptide-producing PP cells; and ghrelin-producing ε-cells.51 These endocrine hormones are secreted into the body via the bloodstream. Pancreatic exocrine function is carried out in acinar cells, which produce amylase and lipase that is secreted into the small bowel via the pancreatic duct.52
Developmentally, the pancreas arises from two separate structures located on either side of the duodenum called the dorsal and ventral buds, both of which arise from the endodermal lining of the duodenum.52 Around the 6th week of fetal development, the ventral bud rotates counterclockwise posterior to the duodenum and fuses with the dorsal bud, with the ducts of the both the dorsal bud and the ventral bud also fusing.51,52 The fused duct now extends the entire length of the pancreas and becomes the main pancreatic duct of Wirsung.51 A smaller remnant of a portion of the prior dorsal bud duct persists as the accessory duct of Santorini.51 In its final configuration, the pancreas retains these two ducts, the main duct (of Wirsung) and the smaller accessory duct (of Santorini) (Fig. 15.23). The main pancreatic duct drains into the duodenum via the major papilla, or ampulla of Vater, whereas the smaller duct drains into the duodenum via the minor papilla.
Anatomically, the pancreas is an elongated, lobular organ located in the retroperitoneum. Growth of the pancreas is accelerated during the first year of life and slows thereafter; thus, the pancreas in a young child is larger relative to body size than in an older child or adult.52 The head of the pancreas and the triangle-shaped uncinate process,
which protrudes posteriorly, are located to the right of midline and are closely associated with the C loop of the duodenum.53 The tail of the pancreas lies to the left and is located near the hilum of the spleen, within the layers of peritoneum that form the splenorenal ligament.53 The pancreas is invested in connective tissue but does not have a true capsule. Arterial supply to the pancreas arises from both the celiac axis and the superior mesenteric artery via the pancreaticoduodenal arteries and branches of the splenic artery.53 Venous drainage from the pancreas is via the splenic vein and other smaller branches that ultimately empty into the portal vein.53
which protrudes posteriorly, are located to the right of midline and are closely associated with the C loop of the duodenum.53 The tail of the pancreas lies to the left and is located near the hilum of the spleen, within the layers of peritoneum that form the splenorenal ligament.53 The pancreas is invested in connective tissue but does not have a true capsule. Arterial supply to the pancreas arises from both the celiac axis and the superior mesenteric artery via the pancreaticoduodenal arteries and branches of the splenic artery.53 Venous drainage from the pancreas is via the splenic vein and other smaller branches that ultimately empty into the portal vein.53
At ultrasound, the normal pancreas appears as a homogeneous structure of slightly increased echogenicity relative to the liver.52 At CT, the gland appears homogenous and similar in attenuation to the liver, with homogeneous enhancement after the administration of intravenous iodinated contrast material. Peak parenchymal enhancement is often in the late arterial phase. At MRI, the pancreas is usually hyperintense compared to liver on T1-weighted pulse sequences.52 The gland is homogeneously isointense to liver on T2-weighted sequences and enhances after intravenous gadolinium-based contrast material administration. The normal main pancreatic duct may or may not be visible at routine imaging. If visible, the main pancreatic duct should measure between 1 and 2 mm and should not be greater than 2.5 to 3 mm in diameter.52
Congenital Abnormalities
Pancreatic Agenesis
Complete pancreatic agenesis occurs when there is complete failure of pancreatic development, resulting in absence of the pancreas. This is a very rare congenital anomaly and is often incompatible with life.54 More frequently, partial pancreatic agenesis occurs, also referred to as pancreatic hypoplasia. In this entity, either the dorsal or ventral pancreatic bud fails to develop; failure of the dorsal bud is more common.54 At imaging, the most common appearance is a prominent, rounded pancreatic head near the duodenum with absence of the pancreatic body and tail.54 There is an association between partial pancreatic agenesis and heterotaxy syndromes with polysplenia54 (Fig 15.24).
Ectopic Pancreas
Ectopic pancreatic tissue is defined as normal pancreatic tissue that is located in an abnormal location and anatomically separate from the normal pancreas. The most common location is the stomach, followed by the duodenum and jejunum55 (Fig 15.25). The reported incidence in autopsy series ranges from 2% to 14%.55,56 Ectopic pancreas is more commonly diagnosed in adults, but has been reported in all ages. The lesions are often small and asymptomatic but can rarely cause complications, such as gastric outlet or bowel obstruction, pancreatitis, or pseudocysts.57
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree