(1)
Thomasville, GA, USA
Abstract
Post-staining of ultrathin sections and/or en bloc staining of specimens is necessary for differential contrast and improved resolution of cellular structures. Often specimens are fixed and stained with osmium tetroxide during fixation, but additional contrast is the result of additional heavy metal stains on the sections. The most common post-staining of sections is done on grids by aqueous uranyl acetate followed by lead citrate. When it is apparent that simple, aqueous uranium and lead post-staining is not adequate, other stains are invoked. These procedures can be as simple as en bloc staining with uranyl acetate after primary fixation and osmication. Over the years, several other treatments have been developed for use with the primary fixation or during dehydration. Tannic acid, paraphenylenediamine (PPD), and malachite green can all serve as en bloc stains and can contribute to overall improved visualization of ultrastructural details in biological specimens. Tannic acid and PPD improve membrane preservation, and malachite green is a phospholipid stain. All of these stains are compatible with aqueous fixatives and should be considered when the usual stains are not satisfactory. Marinozzi rings and microwave-assisted post-staining offer alternatives to traditional grid staining. In addition, stain precipitates on grids often can be removed by treatment with 10 % (v/v) acetic acid.
Key words
EM grids post-stainingMarinozzi ringsMicrowave-assisted stainingEn bloc stainingUranyl acetateLead citrateAlternative stains (tannic acid and paraphenylenediamine)Stain precipitate removal1 Introduction
Staining of biological tissues is needed to improve differential contrast of specimens imaged in the transmission electron microscope (TEM). There is little inherent difference in contrast between embedding media and biological specimens since they are chemically similar in consisting of carbon, hydrogen, oxygen, and other transparent (non-electron dense) atoms (see Fig. 1). Factors that affect contrast in biological TEM are section thickness, the presence of heavy atoms in the specimen, accelerating voltage, and aperture size. Heavy metal stains such as uranium (atomic number 92), lead (atomic number 82), and osmium (atomic number 76) have been used extensively as electron dense stains. Gibbons and Bradfield [1] first used aqueous solutions of lanthanum nitrate and osmium tetroxide in a simple procedure for staining sections on grids for TEM which consisted of floating grids, section side down, in drops of stain. The grids were then washed on drops of distilled water. Watson published on the use of uranium salts [2] to stain tissue sections and introduced the use of lead stains [3]. A number of other lead stains were used in the early 1960s; however, the most reliable lead stain has been the lead citrate stain published by Reynolds in 1963 [4].
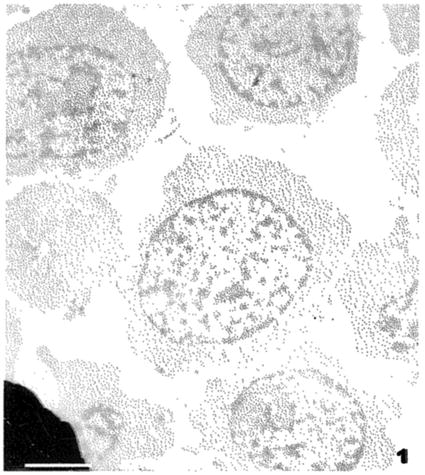

Fig. 1
An unstained section through cultured Jurkat cells. These cells were fixed by an anhydrous protocol and have not been exposed to osmium tetroxide or any other heavy metal or staining. Scale bar = 2.5 μm
Several factors contribute to consistent quality of grid staining. The cleanliness of the work environment during ultramicrotomy and picking up sections on grids as well as the cleanliness of the tools and fluids (see Note 1) used in staining are all extremely important. Several other considerations are the time interval between picking up sections and staining, the embedding medium, and whether or not unstained sections have been exposed to the electron beam. Better contrast is achieved by post-staining grids immediately after the sections are picked up and dried without exposure to the electron beam. If it is necessary to check the quality of sections prior to post-staining, only one grid from a batch should be examined by TEM.
There are numerous methods in the literature and various commercial devices for post-staining grids; the methods presented here are based on keeping the procedures simple and reproducible and use common laboratory materials. The most common staining method in biological TEM consists of post-staining sections on grids with aqueous uranyl acetate followed by Reynolds’ lead citrate [4]. Many stains are general, nonspecific stains; however, certain chemical groups react with uranium and lead ions. Uranium reacts strongly with phosphate and amino groups to stain nucleic acids and phospholipids in membranes. Uranyl acetate stains have a pH of 3.5–4 and strongly stain proteins. An important attribute of uranyl stains is that uranium acts as a mordant for lead stains. This is probably the reason that the post-staining sequence of uranyl acetate followed by lead citrate works so well. Lead binds to negatively charged groups such as hydroxyl groups and areas which reacted with osmium tetroxide such as membranes [5]. Lead stains are highly alkaline and react with carbon dioxide and oxygen to form lead carbonate (PbNO3), a highly insoluble, electron dense precipitate (see Fig. 2). Phosphate groups may be involved in lead staining since use of phosphate buffers often enhances overall staining.
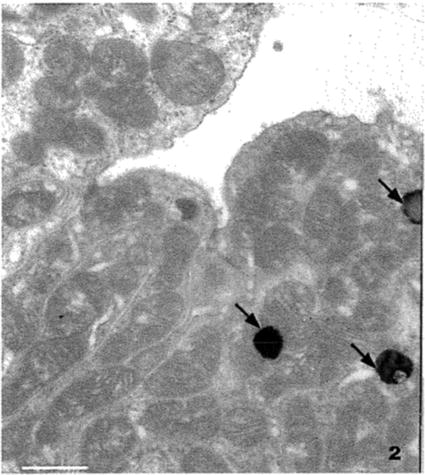

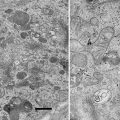
Fig. 2
Section through the convoluted tubules of a dog kidney. The specimen was fixed in Trump’s fixative [35]: 1 % biological grade glutaraldehyde and 4 % formalin (37 % laboratory grade formaldehyde) in sodium phosphate-sodium hydroxide buffer. The tissue was post fixed with osmium tetroxide, dehydrated with methanol, and embedded in a modified Spurr’s epoxy resin [32]. This material was stained with old stains, and the electron dense blobs (arrows) are lead carbonate precipitate. Scale bar = 2.5 μm
Problems related to low contrast and stain precipitation are often related to age of the stains. Old stains loose reactivity when exposed to light and the atmosphere. Prolonged staining times (longer than 10–15 min for uranyl acetate and longer than 5–10 min with lead stains) can result in stain precipitates due to drying at the surface of the drops of stain. Staining at elevated temperatures also contributes to precipitates due to evaporation of the stain solvent. A number of salvage techniques have been developed for removing stain precipitates and restaining grids [6–11]. However, it is better to understand the staining parameters and prevent the formation of precipitates. Thicker sections (gold interference colors) stain faster than thinner sections (gray to silver interference colors) since there are more sites for the stains to react in a thicker section. In addition, contrast can be improved in lightly contrasted sections by using a smaller objective aperture and/or working at a lower accelerating voltage. The trade-off in working at a lower accelerating voltage is the decrease in resolution; however, decreased resolution will probably not be an issue when doing low magnification, survey work.
There are several alternative staining procedures which are useful with difficult specimens. Marinozzi rings [12] (see Fig. 3) were originally prepared for staining sections which required prior oxidation for specialized stains such as silver methenamine or phosphotungstic acid-chromic acid; however, these rings are useful for staining large sections and/or preventing buildup of dried stain near grid bars. Use of the microwave for staining with aqueous uranyl acetate results in improved contrast and shorter staining times [13, 14].
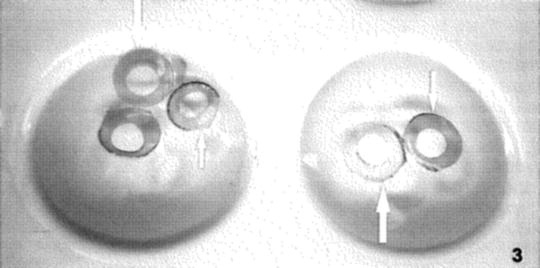

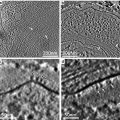
Fig. 3
Marinozzi rings floating on water in wells of a porcelain spot plate. The cut out center of the disc is 3 mm in diameter
Contamination of sections during staining can and does occur when precautions in the avoidance of collection of “dirt” stain precipitates are not adhered to. Salvage techniques have been developed over the years and offer some relief for these problems when it is not practical to start over and do the procedures right the first time [6–11].
In some situations where prolonged staining with uranium and lead is not adequate, it is better to revise the initial specimen preparation and include other components like tannic acid [15, 16] and/or malachite green [17–20] in the primary fixative to stain and to improve the overall structural preservation of some specimens. Paraphenylenediamine (PPD) can be included in the dehydration steps to improve membrane visibility and immunolabel activity [21–24]. Other old protocols include uranyl acetate (0.5–1.0 % w/v) as an aqueous en bloc stain or in the dehydration steps. Certain precautions need to be taken when considering whether or not to use uranyl acetate for en bloc stains (see Note 2). There are numerous other stains which can be done on tissue and grids [25] for specific purposes. The protocols listed above have varying specificities but are often forgotten in our efforts to improve contrast and/or resolution for general morphological studies.
Tannic acid, low molecular weight (see Note 3), is often used in the primary fixative to preserve cell surface components and to improve the delineation of microtubules and basal bodies in cilia and flagella. It is used at 0.5–2.0 % (w/v) in the primary fixative and improves the overall density of membranes and cell surface components. Tannic acid appears to act as a stain mordant for osmium [15, 16].
Specific staining of certain lipid components is often difficult. Malachite green in low concentrations (0.05-0.1% (w/v)) in the primary fixative has been used to preserve waxes on leaf surfaces and lipids in tissues as well as in cell walls of microorganisms [17–19]. Work on Caenorhabditis elegans has demonstrated malachite green to be valuable in identifying lipid components in the cuticle in some strains (see Fig. 4) [20]. Preservation of leaf extracellular wax is also achievable with malachite green.
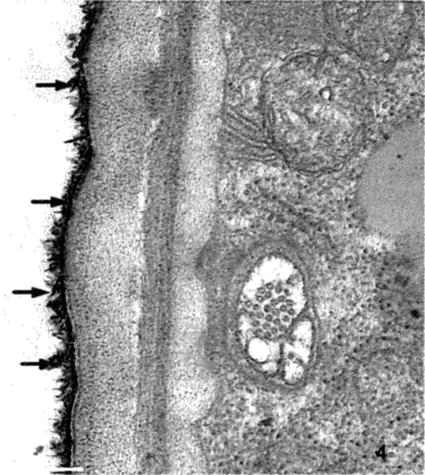

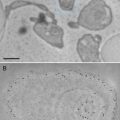
Fig. 4
Section through the cuticle and adjacent muscle layer of C. elegans. This specimen was fixed with 0.1 % malachite green in the primary fixative (glutaraldehyde-paraformaldehyde) followed by osmication. Note the microtubules in the nerve and the detail in the cuticle layers as well as the lipid material on the surface indicated by the extremely electron dense precipitate (arrows) of malachite green with the waxy layer of the cuticle. Scale bar = 500 nm
PPD binds to lipids and improves staining of membranes and other lipid moieties in plant materials such as beta-carotene containing structures in fruits and vegetables. Phendt et al. [21] introduced the use of tannic acid and PPD for an osmium-free preparation for immunolabel, and Brorson et al. [22] have demonstrated improved immunolabel intensity by using 0.1 % (w/v) PPD in dehydration alcohols. PPD can also be used instead of osmium tetroxide as both a stain and a fixative in situations where osmium would interfere with the final specimen analysis [21, 23, 24]. For example, PPD would give excellent structural preservation and analytical results in energy dispersive spectrometry studies when used instead of osmium which can interfere with some peaks. PPD has been used in the author’s lab to replace osmium and improve structural preservation in identifying iron in mitochondrial preparations where osmium or other high Z elements would interfere with identifying the iron peaks by STEM and EDS [24].
2 Materials
2.1 General
1.
Freshly prepared and filtered solution of aqueous uranyl acetate.
2.
5 ml syringes with 0.22 μm filter, two syringes (one syringe with filter for uranyl acetate and another syringe with filter for deionized water).
3.
Parafilm or porcelain spot plates.
4.
Electron microscopy grade forceps.
5.
Glass Petri dishes.
6.
100 ml glass beakers, 4.
8.
Filter paper points.
2.2 Preparation of Staining Solutions
2.2.1 Aqueous Uranyl Acetate
1.
Prepare 2 % (w/v) solution of uranyl acetate [UO2(CH3COO)22H2O] in 5 ml of freshly boiled warm deionized or distilled water (see Note 4). Use a magnetic stirrer and stir until there are no uranyl acetate crystals visible in the bottom of the beaker. It is good idea to cover the top of the beaker with Parafilm. The pH of a freshly prepared 2 % aqueous solution of uranyl acetate is approximately 4.
2.
Filter the solution through Whatman #42 or #50 filter paper.
3.
Store the filtered solution in a dark colored container or wrap the container with aluminum foil to exclude light since uranyl acetate is light sensitive.
4.
Filter an appropriate volume of stain through a 0.22 μm micro filter when ready to stain grids.
2.2.2 Methanolic Uranyl Acetate
(Must use acetone-free methanol; ethanol can be substituted for methanol.)
1.
Prepare a 5 % (w/v) solution of uranyl acetate in 50 % (v/v) methanol. Cover the beaker with Parafilm and stir until there are no uranyl acetate crystals visible in the bottom of the beaker.
2.
Filter and store the solution as described above for aqueous uranyl acetate.
2.2.3 Lead Citrate
1.
In a 50 ml volumetric flask with a glass stopper, place the following components: 1.33 g lead nitrate [Pb(NO3)2], 1.76 g sodium citrate [Na3(C6H5O7) ⋅ 2H2O], and 30 ml of freshly boiled and cooled, deionized, or distilled water.
2.
Stopper the volumetric flask and shake vigorously for 1 min; continue intermittent shaking for 30 min. The solution should be milky.
3.
Add 8 ml of carbonate-free, 1 N NaOH solution (solutions purchased from a reliable vendor work better than freshly prepared NaOH solutions made in the laboratory) (see Note 5). The solution will become clear.
4.




Dilute the solution to 50 ml with freshly boiled deionized or distilled water. The solution of lead citrate is ready for use and should be stored in the glass-stoppered flask or any closed container such that CO2 can be excluded. The final pH of the solution should be about 12.0.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree