Fig. 10.1
Abbreviated timeline of percutaneous coronary intervention and stent development (Courtesy of Richard Schatz, MD)
Stent Design
Integral components to the current and newest generation of stents include stent platform, polymer coating, and antiproliferative drugs.
Stent Platform
Metallurgy
Stents were initially constructed of 316 L stainless steel, composed of iron, chromium, cobalt, and molybdenum, the latter three which are resistant to corrosion. The L indicates the low (<0.03 % m/m) carbon content to prevent formation of chromium carbide, a promoter of corrosion. The backbone for first-generation DES, 316 L stent platforms were not very deliverable due their inflexible nature and high crossing profiles.
Newer generation stents are composed of cobalt, nickel, chromium, and molybdenum that offer increased radial strength and radiopacity [8, 9]. This enabled construction of stents with lower crossing profiles, increasingly thinner struts, flexibility, and hence improved deliverability compared to its stainless steel counterparts. Evidence for these improvements in deliverability was in part reflected in the higher procedural success of thin cobalt chromium to 316 L stainless steel platform DES in the ENDEAVOR III trial (procedure success Endeavor 98.8 % vs. Cypher 94.7 % p = 0.02) [10]. Aside from improved deliverability, thinner struts have reproducibly decreased restenosis rates when compared to thicker bare-metal comparators as demonstrated in the ISAR-STERO studies [11, 12].
Stent Architecture
Current stent architecture is primarily based on the initial slotted tube design by Julio Palmaz [5]. Importance of stent architecture was evident when neointimal hyperplasia and mural thrombus were significantly decreased when stent geometry was matched to the circular vessel lumen instead of distorting it into nonnative geometry [13]. A prospective study randomizing various bare-metal stents showed differences in freedom from myocardial infarction and follow-up angiographic percent diameter stenosis and late loss between different stent designs, reinforcing the concept of optimal stent architecture [14]. The current design of cellular stents is generalized as either open- or closed-cell design (Figs. 10.2 and 10.3). Computer modeling suggests that there is less tissue prolapse with open-cell design and direct comparisons of bare-metal open-cell to closed-cell stents show that the restenosis rates favor open-cell designs [14, 16]. In theory, open-cell designs should allow greater access to side branches and possibly improve patency of covered side branches. Open-cell DES may have advantages with respect to decreasing myocardial infarction (MI) and target lesion revascularization (TLR) when jailing side branches as reported in a post hoc analysis of SPIRIT III [17]. Closed-cell design may enable even distribution of drugs across the endovascular surface, although there is no prospective data indicating this significantly impacts restenosis rates.
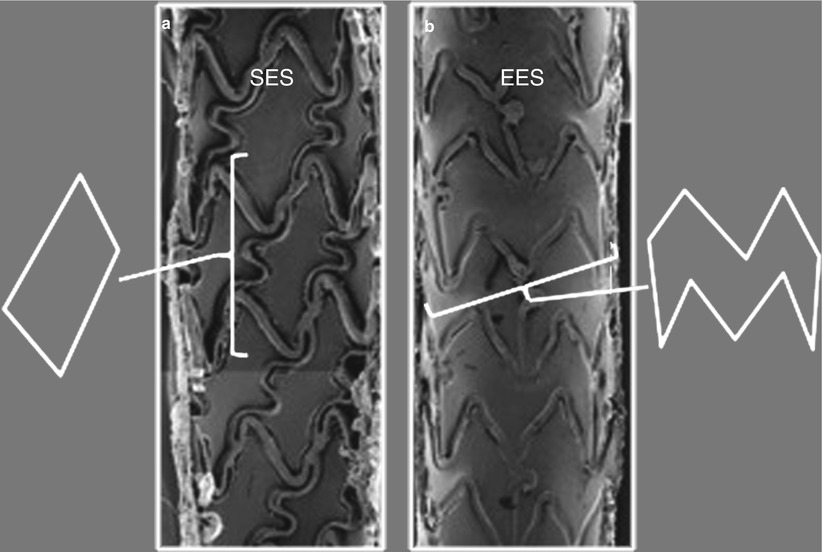
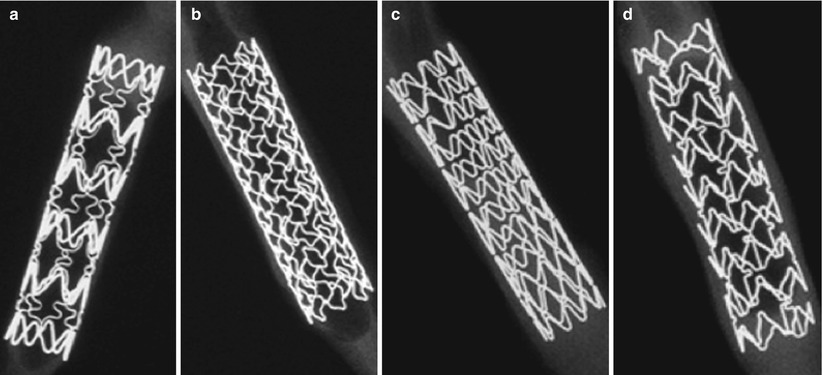

Fig. 10.2
Closed- and open-cell stent design. (a) Closed-cell design of the sirolimus-eluting stent (SES). The cell is enclosed within a simple geometric shape and retains the area regardless of how stretched the stent becomes. (b) Open-cell design of the everolimus-eluting stents (EES). The area enclosed by the cell is more complex in shape with convex and concave inflection points throughout the shape. The cell area is capable of increasing greatly with stent expansion (Adapted from Joner et al. [15], with permission)

Fig. 10.3
High-resolution radiographs of each drug-eluting stent platform. (a) Cypher platform, BX Velocity (Cordis Corp., Johnson ? Johnson, Miami, Florida). (b) TAXUS platform, Liberté (Boston Scientific, Natick, Massachusetts). (c) Endeavor platform, Driver (Medtronic Vascular, Santa Rosa, California). (d) XIENCE platform, Multilink Vision (Abbott Vascular, Santa Clara, California) (Adapted from Joner et al. [15], with permission)
Polymer
Most biological agents require a coating matrix for drug retention and controlling drug-release kinetics. Drugs are released either by particle dissolution or diffusion when polymer breakdown is incorporated (absorbed) into a biodegradable substance. The material should be biologically inert but be able to retain physiochemical composition after sterilization and stent expansion. First-generation DES utilized polymers, long-chained molecules in several small repeating units (Table 10.1) [18]. Although successful in retaining and delivering drug, first-generation polymers have been suspected in causing inflammation implicated in late restenosis and stent thrombosis [19]. An alternate strategy is to improve biocompatibility by using biomimetic compounds. Phosphorylcholine is a naturally occurring zwitterionic compound that emulates a phospholipid membrane that may bind drugs but has a shortened drug elution capacity [20]. Similarly, in an effort to synthetically mimic the phospholipid bilayer structure, the next generation of biocompatible copolymers is Biolinx® (Medtronic, Santa Rosa, CA), a proprietary bled of three polymers: hydrophilic c-19 copolymer, water-soluble polyvinyl pyrrolidinone, and hydrophobic c-10 copolymer [21]. Further details of polymer coatings of individual stents will be discussed further in subsequent sections ascribed to each individual stent.
Table 10.1
Characteristics of drug-eluting stents commercially available in the United States
Cypher | TAXUS | Endeavor | Xience | |
|---|---|---|---|---|
Platform | Bx Velocity | Express/Liberte | Driver | Multilink Vision |
Architecture | Closed cell | Open/open cell | Modular, open cell | Open cell |
Alloy | 316 L | 316 L | Cobalt chromium | Cobalt chromium |
Strut thickness | 140 μm | 132 μm/97 μm | 91 μm | 81 μm |
Drug | Sirolimus | Paclitaxel | Zotarolimus | Everolimus |
Polymer | PEVA/PMBA | SIBS (Translute®) | Phosphorylcholine | VDF-HFP |
Drug elution kinetics | 80 % eluted within 30 days | Slow release 10.2 % released in 10 days | 98 % eluted within 14 days | 80 % eluted within 30 days |
Total elution time | 168 days | Unknown | 21–22 days | 120 days |
Drug
Restenosis remains the chief liability of percutaneous interventions and was the impetus for the development of DES. The pathophysiology of restenosis consists of elastic recoil and negative arterial remodeling, followed by neointimal hyperplasia [22]. Inflammation from the endothelial injury and proliferation of smooth muscle cells are largely responsible for the neointimal hyperplasia; therefore, delivery of antiproliferative agents has been the dominant theme of restenosis therapy. Thus far, the armamentarium of drugs to decrease the proliferative response post-endothelial injury have been derived from chemotherapeutic agents and immunomodulators.
Sirolimus and Limus Analogues
Sirolimus is a macrolide antibiotic derived from cultured Streptomyces hygroscopicus and clinically utilized to prevent transplantation rejection. A potent antifungal, immunosuppressive, and antimitotic agent, sirolimus binds to specific cytosolic proteins instrumental to cell cycle progression (Fig. 10.4). Sirolimus binds to FK506 (FKBP12), and this complex inhibits the activation of mammalian target of rapamycin (mTOR). Binding of mTOR in turn prevents the downregulation of p27, increasing intracellular levels resulting in cyclin-dependent kinase (CDK)-clin complexes. Ultimately arrest of the G1-S phase of the cell cycle halts smooth muscle cell proliferation. Formerly used as an immunosuppressant to treat organ transplant rejection, application towards vascular restenosis was recognized and applied in the Cypher stent [18].
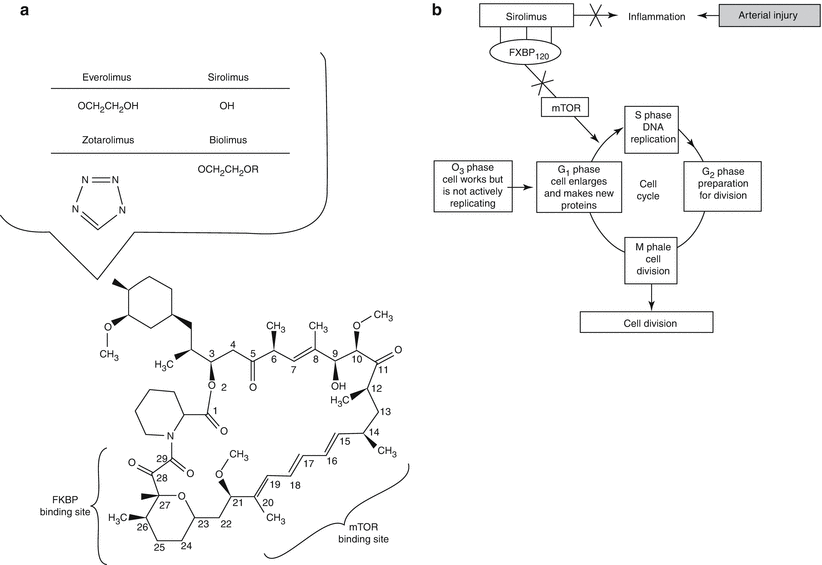

Fig. 10.4
Structure and molecular mechanism of sirolimus and its analogues. (a) Chemical structure of sirolimus showing the FKBP12 and mTOR binding sites. Position 42 is a hydroxyl group in sirolimus but is substituted with various side groups as shown below in other analogues. (b–c) Sirolimus binds to FK-binding protein 12 (FKBP12) and is then enabled to bind to mammalian target of rapamycin (mTOR). mTOR inhibits downregulation of p27kip1, halting the cell cycle between the G1 and S1 phases (Adapted from Garg and Serrrys [23], with permission)
Zotarolimus
Zotarolimus is a proprietary analogue created by substituting a tetrazole ring for the hydroxyl group at position 42 in sirolimus as this site was the most tolerant to modification without altering biological activity (Fig. 10.4). The most hydrophilic of DES drugs, the poor water solubility prevents rapid release into the circulation and favors crossing cell membranes to reach its molecular target [20].
Everolimus
Another analogue of sirolimus, the mechanism of action is identical but its immunosuppressive activity is two to threefold lower than sirolimus in vitro. Nevertheless, in vivo animal data has shown a potent anti-restenotic effect when delivered orally or via DES [18].
Biolimus
Paclitaxel
Isolated from the bark of the Western yew tree, paclitaxel has a complex 8-member ring in its center that stabilizes microtubule formation (Fig. 10.5). Binding to the β-tubulin subunit of microtubules, it antagonizes disassembly and the cell arrests in the middle of mitosis (G2/M phase). Due to its stabilization of microtubules, paclitaxel impacts cell mobility, notably inhibition of smooth muscle cell migration in vitro and in vivo [25].
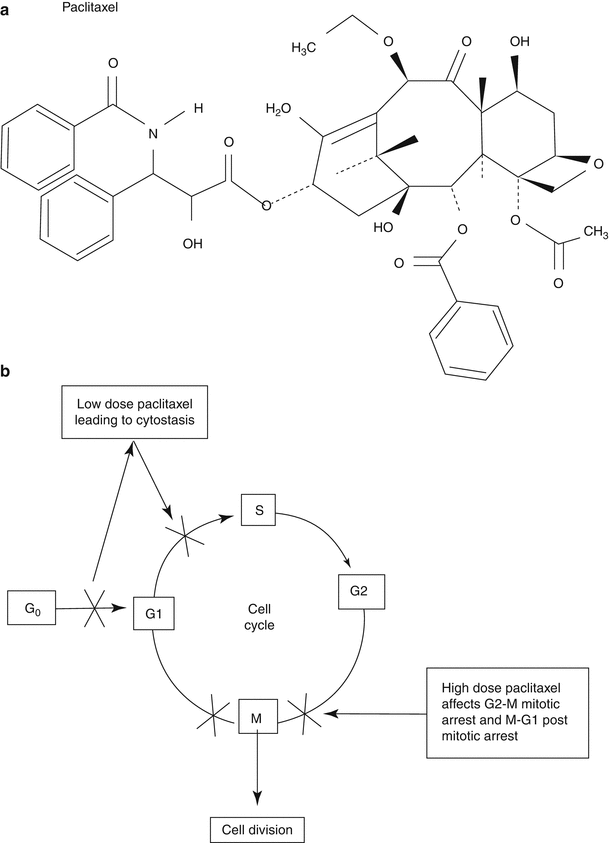

Fig. 10.5
(a) Chemical structure and function (b) of paclitaxel. Paclitaxel stabilizes microtubules resulting in arrest at the mitotic spindle checkpoint of cell division (Adapted from Garg and Serrrys [23], with permission)
Drug-Eluting Stents
Bare-metal restenosis rates of at least 20–25 % in randomized clinical trials prompted the development of strategies to improve the efficacy of stenting [26]. While the mechanical optimization of stenting approached its limits, initial attempts at localized delivery of anti-restenotic medications were unsuccessful due to washout [18]. Stents were appealing as a vehicle for local drug delivery as scaffolding evenly distributed drug to the underlying tissue and served as a reservoir for drug with the possibility of controlling the release kinetics. Harmonizing biocompatibility, drug dose, release kinetics, and drug coating elasticity to yield adequate safety and efficacy proved challenging but at last produced the first clinically efficacious drug-eluting stent, the sirolimus-eluting stent [27].
Sirolimus-Eluting Stents
The Cypher® (Cordis Corporation, Bridgewater, NJ) sirolimus-eluting stent (SES) was the first commercially available DES in the United States in 2003. A 316 L stainless steel platform, the Bx Velocity has relatively thick struts (140 μm) and is a closed-cell stent configuration (Table 10.1). A base coat of PEVA/PMBA (polyethylene-co-vinyl acetate/poly-n-butyl methacrylate) is combined with sirolimus as a base layer while a topcoat is applied to serve as a drug diffusion barrier. Approximately 80 % of sirolimus is released in the first 30 days and becomes undetectable at 168 days [28].
First-in-man experience of SES confirmed durable suppression of neointimal proliferation verified by serial angiographic and intravascular ultrasound over the duration of 12 months laying the foundation for prospective clinical evaluation of SES to treat coronary artery disease [28]. Initial randomized trials RAVEL and SIRIUS proceeded to report consistent decreases in target lesion and vessel revascularization without an increased signal of harm (RAVEL SES TLR 0.0 % vs. BMS 26.6 % P < 0.05) (Table 10.2) [27]. Continued benefit of SES compared to BMS was reproducible across various studies (Table 10.2) in broader patient subgroups including acute myocardial infarction (MI) and more complex coronary artery disease such as long lesions, chronic total occlusions, and diabetes. Analysis of 14 SES vs. BMS trials revealed that SES decreased the risk for the combined endpoint of death, MI, and revascularization (HR 0.43 CI 0.34 to 0.54 P < 0.0001) compared to BMS largely driven by target vessel revascularization [29]. Analysis of stent thrombosis found no significant difference between SES and BMS; however, there was very low rate but trend towards increased late stent thrombosis in patients receiving SES (SES 0.8 % vs. BMS 0.3 % P = 0.16) [29].
Table 10.2
Major clinical trial and pooled analysis results of patients receiving sirolimus-eluting stents (SES) in different clinical settings
Study | Patient population | Follow-up | N | Cardiac death | MI | TLR/TVR | MACE | STa |
|---|---|---|---|---|---|---|---|---|
Kastrati A et al. analysis of 14 trialsb [29] | Pooled analysis | 6–58 months | 14 trials | HR 1.03 | HR 0.93 | HR 0.43 | NA | 0.8 % |
Spaulding C et al. pooled analysis [30] | Pooled analysis | 1,440 days | 878 | 3.5 % | 6.4 % | NA | NA | 3.6 % |
SIRIUSc [31] | Stable CAD, on-label | 5 year | 533 | 8.4 % | 6.2 % | 9.4 % | 20.3 % | 3.9 % |
TYPHOONc [32] | Acute MI, off-label | 4 year | 294 | 2.4 % | 5.2 % | 7.6 % | NA | 5.2 % |
ARTS II [33] | Unrestricted, multivessel disease | 5 year | 607 | 5.4 % | 4.4 % | 14.5 % | 27.2 % | 9.0 % |
RAVELc [34] | Stable CAD, on-label | 5 year | 120 | 12.1 % | 7.3 % | 7.4 % | 25.8 % | 3.3 % |
SCANDSTENT [35] | Unrestricted | 3 year | 163 | 2.5 % | 3.7 % | 4.9 % | 12.3 % | 3.1 % |
J-Cypher [36] | Unrestricted, ACS | 3 year | 2,308 | 7.2 % | 3.3 % | 12.1 % | NA | 2.2 % |
RESEARCH [37] | Unrestricted | 6 year | 508 | 16.3 % | NA | 13.6 % | 29.7 % | 3.1 % |
SESAMIc [38] | Acute MI, off-label | 3 year | 157 | 3.2 % | 2.5 % | 7.0 % | 12.7 % | 5.1 % |
ENDEAVOR IIIc [39] | Stable CAD, on-label | 3 year | 113 | 7.2 % | 4.5 % | 12.2 % | 18.7 % | 1.7 % |
Paclitaxel-Eluting Stents
The TAXUS family of DES has gone through several iterative advances involving the stent platform and polymer/drug formulation. The first-generation commercially available TAXUS Express utilized a 316 L stainless steel platform and was coated with a hydrophobic, elastomeric triblock copolymer (styrene-isobutylene-styrene) commercially known as Translute™ (Boston Scientific, Natick, MA) [40]. Three formulations were developed with increasing drug to polymer ratio from 8.8 to 35 % weight in weight (w/w) at a constant dose density to yield a slow-, moderate-, and fast-release stent coating. The slow-release (TAXUS-SR) drug-polymer combination is commercially available on both the TAXUS Express and TAXUS Liberté stents, while the moderate-release (TAXUS-MR) version of TAXUS was clinically tested in prospective trials, but not made commercially available [41].
TAXUS clinical trial development mirrored that of the Cypher stent. Initial studies determined efficacy of PES in patients with CAD and simple complexity lesions (Table 10.3). TAXUS I showed that PES was indeed superior to BMS with respect to repeat revascularization [49]. TAXUS II compared efficacy of slow-release to moderate-release PES, and while moderate-release PES was found to have lower rates of TLR, it was not commercially developed (5-year TLR TAXUS-SR 10.3 % vs. TAXUS-MR 64.5 %) [42]. The pivotal trial for FDA approval of PES was TAXUS IV, a prospective randomized study comparing TAXUS Express to its identical bare-metal counterpart in treating simple coronary lesions. In short-term and 5-year follow-up, durable efficacy relative to bare-metal stents with respect to TLR was maintained with comparable rates of stent thrombosis between each group (5-year ST BMS 2.1 % vs. TAXUS-SR 2.2 % P = NS) [43].
Table 10.3
Clinical results of patients receiving paclitaxel-eluting stents (PES) for different indications from major studies
Study | Patient population | Follow-up | N | Cardiac death | MI | TLR/TVR | MACE | STa |
|---|---|---|---|---|---|---|---|---|
TAXUS IIb [42] | Stable CAD, on-label | 5 year | 131 | 2.4 % | 4.7 % | 10.3 % | 20.4 % | 2.7 % |
TAXUS IVb [43] | Stable CAD, on-label | 5 year | 643 | 4.4 % | 7.2 % | 9.1 % | 24 % | 2.2 %c |
WDHR [44] | Unrestricted, off-label | 2 year | 1,991 | 3.2 % | 5.3 % | 32 % RRd | NA | 3.3 % |
PASSION [45] | Acute MI, off-label | 5 year | 310 | 8.9 % | 6.8 % | 7.7 % | 18.6 % | 4.2 % |
HORIZONSb [46] | Acute MI, off-label | 3 year | 2,257 | 3.2 % | 7.0 % | 9.4 % | 20.0 % | 4.8 %c |
SPIRIT IIIb [47] | Stable CAD, on-label | 3 year | 332 | 1.9 % | 6.6 % | 12.8 % | 16.4 % | 1.7 %c |
ENDEAVOR IVb [48] | Stable CAD, on-label | 3 year | 775 | 2.4 % | 4.9 % | 6.1 % | 13.6 % | 3.0 % |
T-SEARCH [37] | Unrestricted, off-label | 6 year | 576 | 16.0 %e | NA | 12.5 % | 29.9 % | 2.8 % |
In order to enhance procedural performance, paclitaxel and Translute were affixed to a different stainless steel platform, the Liberté™ (Natick, MA, Boston Scientific) stent. The Liberté is a 316 L stainless steel platform with significantly thinner struts (Express 132 μm vs. Liberté 97 μm) and a more uniform strut pattern to improve distribution of drug. Subsequently, TAXUS Liberté was clinically tested in the TAXUS ATLAS prospective trial, comparing patients receiving TAXUS Liberté to historical controls pooled from TAXUS IV and V trials [50]. Overall, the TAXUS Liberté platform met its endpoints for non-inferiority compared to the TAXUS Express stent at 9-month follow-up; however, in a prespecified subgroup analysis, TAXUS Liberté treatment of small vessels was superior to TAXUS EXPRESS in reducing 9-month TLR in small vessels (16.9 % vs. 6.1 % P < 0.05) [51].
Expansion of PES utilization in the setting of acute MI showed consistent results in decreased TLR rates compared to bare-metal counterparts (PES 4.5 % vs. BMS 7.5 % P = 0.002) in HORIZONS-AMI, a multicenter trial with a 2 × 2 factorial design randomizing against bivalirudin and heparin/glycoprotein IIb/IIIa inhibitors and PES vs. BMS [52, 53]. Notably, stent thrombosis for both BMS and PES remained relatively high for each cohort (ST PES 3.2 % vs. BMS 3.4 % P = 0.77) at 1 year [54].
Sirolimus-Eluting Stents vs. Paclitaxel-Eluting Stents
After the release of both first-generation DES, head to head comparisons were drawn as both stents had contrasting drug mechanisms, elution kinetics, and polymers. Direct comparisons in patients with stable CAD in real-world context were performed in the SIRTAX trial comparing SES and PES [55]. Angiographic follow-up showed increased late lumen loss and binary restenosis translating into increased TLR for the PES group (Table 10.4). Increased late loss and binary restenosis were again observed in a trial with simpler lesion subsets, the REALITY trial, signaling that PES intrinsically may carry greater risks of in-stent restenosis [61]. Despite higher rates of binary restenosis, no significant clinical impact was observed as similar rates of TLR occurred in both SES and PES cohorts (TLR SES 6.0 % vs. PES 6.1 % P > 0.99) [61]. Based on the less favorable angiographic restenosis seen in studies with angiographic follow-up, expectation for greater separation in TLR events between SES and PES would have been expected in diabetic populations. While PES was found to have more in-stent restenosis (ISR) in the DIABETES trial (TLR SES 2.0 % vs. PES 7.5 % P = 0.017) [63], equivalent performance with respect to major adverse cardiac events (MACE) and target lesion revascularization/target vessel revascularization (TLR/TVR) was seen in subsequent head to head SES/PES trials for different lesion subsets including randomized comparison for treating bare-metal or SES restenosis (Table 10.4) [59, 64]. A nonrandomized comparison of SES and PES in a real-world context was published from the Rotterdam DES registries also showing no significant difference in terms of MACE [37]. However, when the data is pooled in meta-analysis, PES was found to have an increased hazard towards myocardial infarction, TLR/TVR, and stent thrombosis compared to SES utilization (Table 10.4) [62].
Table 10.4
Results of major studies comparing the performance of sirolimus-eluting stents (SES) and paclitaxel-eluting stents (PES) against the background of different clinical indications
Study | Stent | Follow-up | N | MACE | MI | TLR/TVR | Death | ST | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
% | P | % | P | % | P | % | P | % | P | ||||
SORT OUT II [56] | SES | 546 d | 1,065 | 10 | 0.21 | 4.2 | 0.32 | 4.5 | 0.14 | 1.7 | 0.8 | 2.6 | 0.7 |
PES | 1,033 | 11.6 | 5.1 | 5.9 | 1.5 | 2.8 | |||||||
SIRTAX [55] | SES | 9 mo | 503 | 6.2 | 0.009 | 2.8 | 0.49 | 4.8 | 0.03 | 0.6 | 0.15 | 2.0 | 0.62 |
PES | 509 | 10.8 | 3.5 | 8.3 | 1.6 | 1.6 | |||||||
Diabetes [57] | SES | 2 year | 200 | 3.5 | 0.001 | 0.5 | 0.999 | 3.5 | 0.004 | 0 | 0.248 | 1.0 | 0.499 |
PES | 200 | 12.5 | 1.0 | 11.0 | 1.5 | 0 | |||||||
ISAR DIABETES [58] | SES | 9 mo | 125 | NA | NA | 4.0 | 0.72 | 6.4 | 0.13 | 3.2 | 0.52 | NA | NA |
PES | 125 | NA | 2.4 | 12.0 | 4.8 | NA | |||||||
ISAR DESIRE [59] | SES | 1 year | 100 | NA | NA | 1.0 | NS | 8.0 | 0.02 | 2.0 | NS | NA | NA |
PES | 100 | NA | 2.0 | 19.0 | 1.0 | NA | |||||||
ISAR DESIRE 2 [60] | SES | 1 year | 225 | 20.4 | 0.71 | 2.7 | 0.53 | 16.6 | 0.52 | 3.4 | 0.60 | 0.4 | 0.67 |
PES | 225 | 19.6 | 1.8 | 14.6 | 4.5 | 0.4 | |||||||
RESEARCH [37] | SES | 6 year | 508 | 29.7 | HR 1.01 | NA | NA | 13.6 | HR 1.06 | 16.3 | HR 1.0 | 3.1 | 0.9 |
PES | 576 | 29.9 | (0.9–1.14) | NA | 12.5 | (0.89–1.26) | 16.0 | (0.86–1.17) | 2.8 | ||||
REALITY [61] | SES | 1 year | 684 | 10.7 | 0.73 | 5.1 | 0.55 | 6.0 | 0.99 | 1.5 | 0.63 | NA | NA |
PES | 669 | 11.4 | 6.0 | 6.1 | 1.0 | ||||||||
Meta-analysis [62] | SES | HR 0.84 | HR 0.74 | HR 0.92 | HR 0.66 | ||||||||
PES | (0.69–1.03) | (0.63–0.87) | (0.74–1.13) | (0.46–0.94) |
On-Label vs. Off-Label Use of DES
The approval of both first-generation drug-eluting stents was based on clinical trials enrolling symptomatic patients treating vessel diameters between 2.5 and 3.5 mm and discrete lesions less than 30 mm in length. These patients did not represent high-risk clinical indications (e.g., acute coronary syndromes) or complex lesion morphologies and therefore constitute the most stable segment of the patient spectrum undergoing PCI yet only make up an estimated 51 % of patients of the NHLBI registry referred for stent implantation [65]. Off-label indications include higher-risk patient and lesion subsets such as acute myocardial infarction, chronic total occlusions, saphenous vein graft lesions, bifurcations, and long stenoses (Table 10.5). The remaining 49 % of patients in the NHLBI Dynamic Registry treated for off-label indications and predictably TVR, MI, stent thrombosis, and death are consistently worse in the off-label cohort (Fig. 10.6) [66]. The efficacy of DES compared to BMS in off-label use mirrors on-label DES use with improvements in repeat revascularization, but the analysis showed decreased rates for death and MI for DES use (Table 10.6) [65]. High atherosclerotic burden, selection bias towards higher-risk patients (acute myocardial infarction), and more complex anatomy (chronic total occlusions) temper the effectiveness of DES. For instance, in the E-Five Registry, comparison of the on- and off-label use showed increases in stent thrombosis (all ARC ST on-label 0.8 % vs. off-label 2.2 % P < 0.001), myocardial infarction (MI on-label 0.7 % vs. off-label 1.9 % p < 0.001), and cardiac death (cardiac death 0.9 % vs. 2.2 % p < 0.001) (Table 10.7) [69].
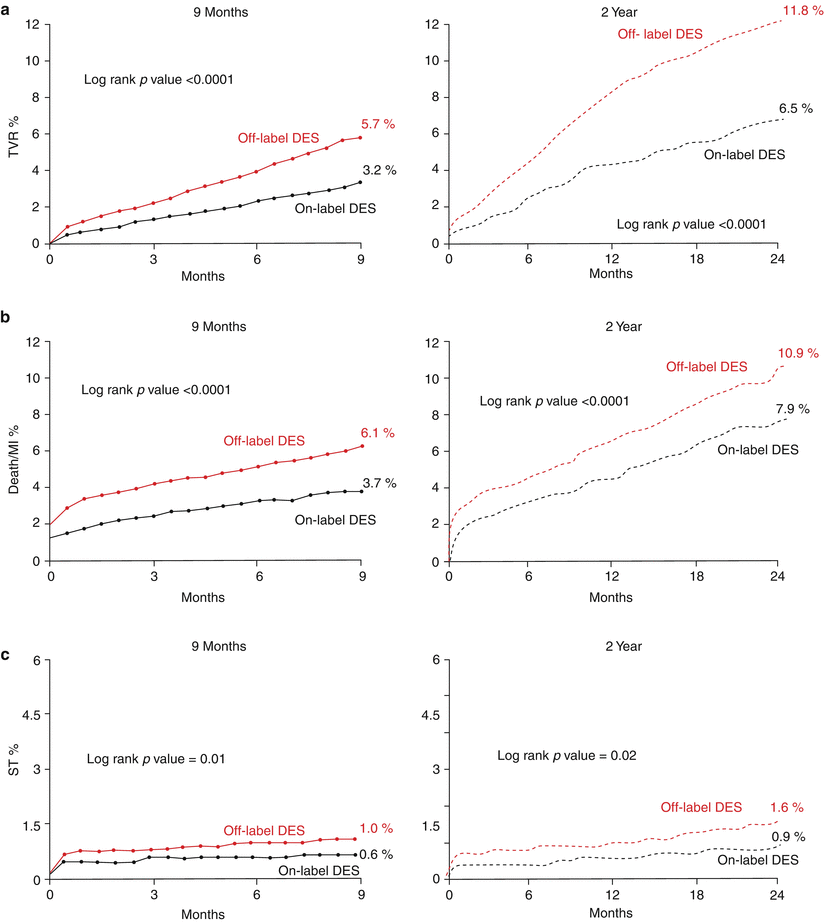
Table 10.5
Definitions of on-label and off-label use of drug-eluting stents
On-label indications for DES |
Patients with symptomatic CAD of inducible ischemia |
Discrete de novo coronary lesions |
Vessel diameter ≥2.5 mm to ≤3.5 mm |
Lesion length ≤30 mm |
Off-label indications for DES |
ST-elevation myocardial infarction |
Chronic total occlusions |
Saphenous vein grafts |
Bifurcation lesions |
Ostial lesions |
Left main stenosis |
In-stent restenosis |
Long lesions |
Small vessels |
Large vessels |
Multi-lesion percutaneous coronary intervention |

Fig. 10.6
Comparison of event rates between off-label and standard use of drug-eluting stents (DES). Kaplan-Meier estimates comparing event rates at 9 months and 2 years between off-label and on-label use of DES in the STENT (Strategic Transcatheter Evaluation of New Therapies) group, a US multicenter prospective registry. (a) Target vessel revascularization (TVR), (b) death or myocardial infarction (death/MI), and (c) stent thrombosis (From Brodie et al. [66], with permission)
Table 10.6
Cumulative 1-year rates of safety and efficacy of bare-metal and drug-eluting stents in the NHLBI Dynamic Registry [65]
Standard | Off-label | |||||
|---|---|---|---|---|---|---|
Bare-metal stent | Drug-eluting stent | Bare-metal stent | Drug-eluting stent | |||
N = 1,748 | N = 1,381 | N = 2,110 | N = 1,312 | |||
% | p-Value | % | p-Value | |||
Safety | ||||||
Death | 2.7 | 2.8 | 0.88 | 6.4 | 3.7 | <0.001 |
Myocardial infarction | 4.1 | 3.3 | 0.24 | 5.9 | 4.4 | 0.06 |
Death or myocardial infarction | 6.4 | 5.8 | 0.42 | 11.6 | 7.5 | <0.001 |
Efficacy | ||||||
Repeat percutaneous coronary intervention | 10.5 | 6.5 | <0.001 | 13.6 | 11.4 | 0.07 |
Coronary artery bypass grafting | 4.3 | 1.4 | <0.001 | 5.1 | 1.5 | <0.001 |
Repeat revascularization | 13.4 | 7.7 | <0.001 | 17.5 | 12.7 | <0.001 |
Table 10.7
Compiled results of patients receiving zotarolimus-eluting stents (ZES) in major clinical trials and registries
Study | Patient population | Follow-up | N | Cardiac death | MI | TLR/TVR | MACE | STa |
|---|---|---|---|---|---|---|---|---|
ENDEAVOR I [67] | Stable CAD, on-label | 12 month | 100 | 0.0 % | 1.0 % | 1.0 % | 2.0 % | 1.0 % |
ENDEAVOR IIb [68] | Stable CAD, on-label | 270 day | 592 | 1.2 % | 2.7 % | 4.6 % | 7.3 % | 0.5 % |
ENDEAVOR IIIb [10] | Stable CAD, on-label | 3 year | 323 | 3.3 % | 0.6 % | 17.9 % | 18.6 % | 0.9 % |
ENDEAVOR IV [48] | Stable CAD, on-label | 3 year | 773 | 1.7 % | 2.1 % | 6.5 % | 11.3 % | 2.2 % |
E-Five Registry [69] | Standard use, on-label | 12 month | 2,125 | 0.9 % | 0.7 % | 2.8 % | 4.3 % | 0.8 % |
E-Five Registry [69] | Unrestricted, off-label | 12 month | 6,189 | 2.0 % | 1.9 % | 5.0 % | 8.6 % | 2.2 % |
SORT OUT III [70] | Unrestricted, off-label | 18 month | 1,162 | 2.0 % | 2.0 % | 6.0 % | 10.0 % | 1.0 % |
Zotarolimus-Eluting Stents
Zotarolimus-eluting stents (ZES) (Endeavor®, Medtronic Vascular, Santa Rosa, California) are composed of a cobalt chromium (Driver®, Medtronic) platform. The stent is coated with phosphorylcholine, a blended composite polymer primarily comprised of hydrophilic monomers engineered to mimic the chemical structure of phospholipid head groups. A basecoat of phosphorylcholine is applied to the stent followed by a layer of zotarolimus, which then overlaid with a final coat of phosphorylcholine. A unique feature of ZES stents is rapid eluting of zotarolimus as 90 % is dispersed within 14 days and should remain within the vessel for 28 days. Zotarolimus (ABT-578, Abbott Pharmaceuticals, Abbott Park, Il) is a tetrazole-containing macrocyclic immunosuppressant that shares structural similarity and biological activity with sirolimus (Fig. 10.4).
Clinical testing of ZES first began in 2003 with ENDEAVOR I trial, a single-arm prospective observational study conducted in patients with stable CAD evaluating the medium- and long-term safety and efficacy in this first-in-man trial. With a low MACE rate of 2 % and 1 year TLR rate of 1 %, this paved the way for subsequent trials (Table 10.7) [67].
Zotarolimus-Eluting Stents vs. Bare-Metal Stents
A prospective multicenter trial outside the United States, the ENDEAVOR II randomized 1,197 patients to ZES or its corresponding Driver bare-metal stent platform (Table 10.7). This study confirmed that ZES successfully decreases rates of TLR and TVR compared to its bare-metal platform without any increase in death, MI, or stent thrombosis [68]. Angiographic and IVUS 8-month follow-up showed that late loss and binary restenosis consistently favored ZES compared to BMS. With regard to the overall safety and efficacy compared to bare-metal stents, a pooled analysis of six studies with a mean follow-up of 4.1 years comparing ZES and BMS in 2,132 and 596 patients, respectively, demonstrated equivalence in outcomes with respect to hard endpoints and a 58 % reduction in TLR in patients implanted with ZES compared BMS. A small nonsignificant increase in Academic Research Consortium (ARC)-defined definite/probable stent thrombosis favoring ZES (ZES 0.8 %; BMS 1.7 % P = NS) was observed, signaling that the lower stent thrombosis risk in ZES may confer an advantage to first-generation DES counterparts [71] (Table 10.8).
Table 10.8
Academic Research Consortium (ARC) definitions for stent thrombosis
Academic Research Consortium definitions of stent thrombosis |
|---|
Definite |
Angiographic evidence of thrombosis |
Pathological confirmation of stent thrombosis |
Probable |
Any unexplained death within first 30 days |
Irrespective of time after the index procedure, any MI that is related to the documented acute ischemia in the territory of the implanted stent without angiographic confirmation of stent thrombosis |
Possible stent thrombosis |
Clinical definition of possible stent thrombosis is considered to have occurred with any unexplained death from 30 days after intracoronary stenting until the end of follow-up |
Acute stent thrombosis |
0 to 24 h after stent implantation |
Subacute stent thrombosis |
>24 h to 30 days after stent implantation |
Late stent thrombosis |
>30 days to 1 year after stent implantation |
Very late stent thrombosis |
>1 year after stent implantation |
Zotarolimus-Eluting Stents Compared to Other Drug-Eluting Stents
Clinical development of ZES continued with a non-inferiority comparison of ZES to SES in ENDEAVOR III. Evaluating a primary endpoint of angiographic in-segment late lumen loss at 8 months, the study did not achieve non-inferiority, demonstrating significantly higher late lumen loss and restenosis with the Endeavor stent despite no significant differences in repeat revascularization (SES 0.13 ± 0.32 mm vs. ZES 0.34 ± 0.44 mm P < 0.001) [10]. Differences in rate of non-Q-wave MI favoring ZES (ZES 0.6 % vs. SES 3.5 % P = 0.04) were observed largely driven by post-procedure myonecrosis. In spite of initially higher angiographic late lumen loss, rates of clinical restenosis beyond the period of protocol-specified angiographic follow-up remained stable with ZES compared with SES, resulting in similar late-term efficacy [73]. Over 5 years, significant differences in death, MI, and composite endpoints favored treatment with ZES, contesting the notion that less favorable early angiographic surrogates of efficacy accurately predict important clinical events.
ENDEAVOR IV compared ZES to PES in standard de novo coronary stenosis (Table 10.6). ZES compared favorably with PES, with regard to MACE at 1 year, but after extending the follow-up to 3 years, a significant difference in rates of myocardial infarction was observed (ZES 2.1 % vs. PES 4.9 % p = 0.005) [39]. This was attributed to increased rates in very late stent thrombosis (VLST) observed in the PES cohort (definite/probable ST ZES 0.1 % vs. PES 1.6 % p = 0.004) with a minimal increase of VLST in ZES during the 2nd and 3rd year of follow-up. Again primary angiographic endpoint at 8 months in the trial showed greater late lumen loss and binary restenosis for ZES; however, this did not confer higher rates of TLR at the 3-year assessment [48, 74].
Zotarolimus-Eluting Stents in Routine Clinical Practice
Results of ZES in routine clinical practice outside stringent clinical trial restrictions on lesion type or patient clinical risk had yet to be tested (e.g., STEMI); therefore, greater than 8,000 patients in post-marketing surveillance were followed for safety and efficacy across a broad spectrum of lesions and patient groups. The 12-month clinical outcomes for ZES implantation showed that implantation in an off-label fashion yielded favorable but less comparable results to on-label utilization of ZES. For instance, higher rates of stent thrombosis (definite/probable ST off-label 1.4 % vs. on-label 0.4 %) and TLR (off-label 5 % vs. on-label 2.8 %) were observed, suggesting that low thrombosis rates previously observed in simple and moderately complexity disease in the ENDEAVOR III/IV trials could not be assumed when treating unselected patients with complex coronary disease [69].
Effectiveness of ZES and SES were directly compared in routine clinical practice in the randomized SORT OUT III trial. A total of 2,332 patients were randomized to receive ZES or SES and followed clinically for 18 months. At 18 months, patients receiving ZES experienced higher rates of TLR (ZES 6 % vs. SES 2 % P < 0.0001). A slightly higher rate of myocardial infarction was observed in the ZES group (ZES 2 % vs. SES 1 % P = 0.029), although rates of stent thrombosis were similar between groups at 18 months [70].
New-Generation Zotarolimus-Eluting Stents
The latest generation of zotarolimus-eluting stents is the RESOLUTE® DES (Medtronic Vascular, Santa Rosa, California) which utilizes the identical Driver® platform and zotarolimus drug but has a different polymer. The Biolinx® (Medtronic Vascular, Santa Rosa, California) polymer is a blend of three polymers: hydrophilic c-19 copolymer, water-soluble polyvinyl pyrrolidinone, and hydrophobic c-10 copolymer [21]. This new polymer extends drug elution to 180 days total in attempts to decrease restenosis rates. RESOLUTE stents showed equivocal outcomes compared to EES with respect to death, ischemia-driven TLR, myocardial infarction, and overall MACE through the 2 years of follow-up (Tables 10.9 and 10.10) in the RESOLUTE ALL COMERS trial. The first year reported rates of definite stent thrombosis favored EES (definite ST RESOLUTE 1.2 % vs. EES 0.3 % p = 0.01) as did combined definite/probable stent thrombosis rates (definite/probable ST RESOLUTE 1.6 % vs. EES 0.7 % P = 0.05) [76]. This combined rate of definite/probable stent thrombosis remained numerically higher for the RESOLUTE stent cohort at 2 years but was no longer significantly different (definite/probable ST RESOLUTE 1.9 % vs. EES 1.0 % P = 0.077) [77]. The RESOLUTE-US study was prospective observational trial enrolling 1,402 patients with stable CAD and demonstrated lower of stent thrombosis relative to RESOLUTE ALL COMERS study (definite/probable ST 0.1 %), likely related to high proportion of patients with myocardial infarction (33–34 %) enrolled in the ALL COMERS study, while the US study excluded acute coronary syndromes [78].
Table 10.9
Clinical results of zotarolimus-eluting Resolute® (Medtronic Cardiovascular, Santa Rosa, California) stents
Study | Patient population | Follow-up |
|---|