Diagnostic modalities for the diagnosis of acute stroke have increased in number and quality. Magnetic resonance imaging has increasingly become a central tool for the management of patients with stroke. New sequences, such as diffusion and perfusion, provide insight into the infarcted core and the hypoperfused brain. The use of higher magnetic fields allows us to gain in signal strength, which can be used to improve imaging speed and/or resolution. Recent additional sequences allow perfusion without contrast and susceptibility-weighted imaging can help identify early bleeding. These new techniques should provide more information about the on going ischemic process.
- •
Imaging must be fast and comprehensive (20 mins).
- •
Exclude hemorrhage with T2∗/susceptibility-weighted imaging.
- •
Demonstrate/exclude ischemia with diffusion-weighted imaging/diffusion tensor imaging.
- •
Demonstrate tissue at risk with perfusion.
- •
Demonstrate occlusion with time of flight.
- •
Demonstrate/exclude proximal carotid disease with neck magnetic resonance angiography.
When confronted with a patient with acute stroke symptoms:
Rule out other pathology (tumor, inflammation, hemorrhage)
Demonstrate ischemia
Demonstrate cause of stroke
Identify other possible cause of stroke (venous)
Demonstrate potentially viable tissue (penumbra)
Imaging techniques: CT versus MR imaging
As already mentioned, the 2 main aims of neuroimaging are excluding hemorrhage and demonstrating ischemia. CT has shown itself to be very sensitive to hemorrhage and also capable of detecting signs of early ischemia in well-trained hands. Using MR imaging, these 2 aims can be attained by using T2∗ images for blood detection and diffusion-weighted imaging (DWI) for ischemic lesions. Although initially there was a heated debate if MR could fulfill the first imaging criterion (exclusion of hemorrhage), we have now seen that this is easily feasible with MR imaging. Although in a few select situations, such as subarachnoid hemorrhage, which usually does not mimic stroke clinically, imaging can be done with both MR imaging and CT for screening of hemorrhage; MR imaging is ideally suited for the detection of ischemia. MR imaging with diffusion has been shown to be able to detect strokes with sensitivities as high as 90% or more. The reports by Kidwell and colleagues and Chalela and colleagues found MR imaging and CT to be equivalently suited for the detection of acute hemorrhage. Multiple studies comparing CT and MR imaging have shown that diffusion imaging is extremely sensitive to the presence of infarction. Although CT criteria are well established and have been used with success to detect early ischemic changes, MR imaging can much better detect the presence of small subcortical and cortical infarcts.
MR imaging protocols now run for approximately 20 to 30 minutes, meaning that imaging time will not interfere to a greater degree anymore. This time allows for performance of diffusion imaging, T2∗ imaging, and T2 imaging, as well as MR angiography (MRA) and MR perfusion ( Box 1 ).
Minimal stroke protocol:
Axial diffusion-weighted imaging (b0 and b max)
Axial T2∗ images
Intracranial MR angiography (time of flight)
Regular stroke protocol:
Add to above:
Axial T1-weighted images
Axial T2-weighted images
Contrast-enhanced perfusion imaging
Extensive stroke protocol:
Add to above:
Extracranial and intracranial contrast-enhanced MR angiography of the neck
Although being initially very sensitive to lesion detection, MR protocols, including diffusion imaging, have also shown themselves to provide information about outcome and lesion progression, as well as provide information about possible cause of ischemia owing to pattern differences in lesion location.
MR imaging also has established itself as the post-therapeutic modality of choice. This is because DWI can be performed that is able to demonstrate very small lesions that might occur after treatment, as well as demonstrate reperfusion with apparent diffusion coefficient (ADC) mapping.
Considering all these arguments, it is obvious why MR imaging is destined to become the method of choice for stroke management.
When considering the guidelines of the American Heart Association, we can see that CT and MR imaging can be used to exclude pathology and demonstrate ischemia, that angio-MR imaging can detect the presence of occlusion as well or almost as well as other concurrent techniques, such as CT angiography or digital subtraction angiography, and that perfusion imaging, although not yet fully validated, can demonstrate the presence of hemodynamic changes that correlate well with the neurologic changes that will eventually predict outcome. Thus, the use of multimodality imaging is today necessary and this is rendered especially easy at a high field.
Diffusion imaging
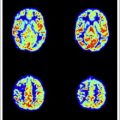
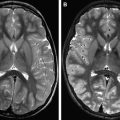
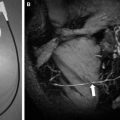
To the diffusion-imaging techniques belong DWI and diffusion tensor imaging. DWI is mainly used for stroke and consists of a relatively simple modification of a spin-echo sequence that is sensitized to motion by diffusion gradients. This produces the so-called diffusion-weighted images; at the higher b value, early ischemia will lead to a hyperintensity on imaging that corresponds to stroke. DWIs at a high b value have an inherent strong contrast and any ischemic lesion will appear as a bright signal against a dark background ( Fig. 1 ). Nowadays, mostly directly isotropic images are used and produced : images without artifacts owing to the directionality of water motion seen when the gradients are used with different directions. Usually DWI is done at various settings of diffusion sensitivity (b values) and these various images are then used to create the so-called maps of the ADC; whereas the DWIs are used for screening purposes and detection, the ADC maps provide an interesting way to quantify or date the ischemic event. Because of their strong inherent contrast, DWIs are better suited to detect also small ischemic events, such as those caused by hemodynamic compromise or embolism ( Fig. 2 ). Although the diffusion effects themselves are independent of the magnetic field, the use of a higher field will improve quality. In a study comparing DWIs at 1.5 and 3 T in 25 patients, Kuhl and colleagues found an increased diagnostic confidence provided by the higher field images when compared with the standard 1.5 T images, despite the presence of higher susceptibility artifacts but principally because of higher signal-to-noise ratio (SNR). Also, diffusion imaging is ideally suited for the follow-up of patients who have undergone interventional procedures ( Fig. 3 ).
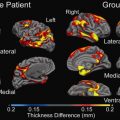
Diffusion tensor imaging may at the moment have a less clearly established role in the ultra-early assessment of patients with stroke; indeed diffusion images with more directionality have a slightly lesser contrast and are often less used clinically. The maps of fractional anisotropy, which reflect directionality, have a rather low imaging resolution so they are rarely used for the diagnosis of acute stroke; however, in the follow-up after the ischemic event, the use of tractography as provided by diffusion tensor imaging could be of great interest, as it allows us to follow the impact of the stroke on connectivity ( Fig. 4 ). Diffusion tensor imaging will benefit greatly from the use of higher magnetic fields because it will increase the SNR greatly.
Diffusion imaging
To the diffusion-imaging techniques belong DWI and diffusion tensor imaging. DWI is mainly used for stroke and consists of a relatively simple modification of a spin-echo sequence that is sensitized to motion by diffusion gradients. This produces the so-called diffusion-weighted images; at the higher b value, early ischemia will lead to a hyperintensity on imaging that corresponds to stroke. DWIs at a high b value have an inherent strong contrast and any ischemic lesion will appear as a bright signal against a dark background ( Fig. 1 ). Nowadays, mostly directly isotropic images are used and produced : images without artifacts owing to the directionality of water motion seen when the gradients are used with different directions. Usually DWI is done at various settings of diffusion sensitivity (b values) and these various images are then used to create the so-called maps of the ADC; whereas the DWIs are used for screening purposes and detection, the ADC maps provide an interesting way to quantify or date the ischemic event. Because of their strong inherent contrast, DWIs are better suited to detect also small ischemic events, such as those caused by hemodynamic compromise or embolism ( Fig. 2 ). Although the diffusion effects themselves are independent of the magnetic field, the use of a higher field will improve quality. In a study comparing DWIs at 1.5 and 3 T in 25 patients, Kuhl and colleagues found an increased diagnostic confidence provided by the higher field images when compared with the standard 1.5 T images, despite the presence of higher susceptibility artifacts but principally because of higher signal-to-noise ratio (SNR). Also, diffusion imaging is ideally suited for the follow-up of patients who have undergone interventional procedures ( Fig. 3 ).
Diffusion tensor imaging may at the moment have a less clearly established role in the ultra-early assessment of patients with stroke; indeed diffusion images with more directionality have a slightly lesser contrast and are often less used clinically. The maps of fractional anisotropy, which reflect directionality, have a rather low imaging resolution so they are rarely used for the diagnosis of acute stroke; however, in the follow-up after the ischemic event, the use of tractography as provided by diffusion tensor imaging could be of great interest, as it allows us to follow the impact of the stroke on connectivity ( Fig. 4 ). Diffusion tensor imaging will benefit greatly from the use of higher magnetic fields because it will increase the SNR greatly.