The symbol, X, can be replaced by a unique identifier (chemical symbol) to remove the need for Z to be stated, e.g. 12C to represent carbon (A=12, Z=6). An isotope of an element contains the same number of protons (Z), but a different number of neutrons (A–Z). Many elements appear naturally in the form of more than one isotope, some of which may be radioactive. As it is the atomic number that largely governs the chemical behaviour of elements (due to the arrangement of electrons discussed below), a stable element and its radioactive isotope will behave identically chemically. This feature can be made use of for clinical investigations, such as monitoring the uptake of iodine in the thyroid using radioactive 131I as opposed to the stable isotope of 127I.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


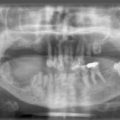
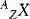 1.3
1.3