Inclusion criteria
COPD with emphysema with severe irreversible obstruction to airflow
FEV1 < 35% pred
Marked hyperinflation of the lung
TLC > 110% pred
Impaired exercise performance
RV > 200%
RV/TLC > 0.65
6′ walking < 350 m
Exclusion criteria
Pulmonary hypertension
PAPm > 35 mmHg
Hypercapnia*
paCO2 > 55 mmHg*
“Destroyed lung” anda
DLCO < 20% * and
aHomogeneous emphysema
aHomogeneous emphysema
However, in our experience, many patients with advanced emphysema consider an improvement in dyspnoea, exercise capacity and quality of life of more importance than the prolonged survival per se. It is obvious that resection of functionless tissue such as in heterogeneous emphysema with or without bullae can be advised to the patient with a relative low risk of mortality and a high chance of significant improvement in dyspnoea, walking distance, quality of life and lung function. FEV1 improves in a range of 40–80% from baseline with a peak at 3–6 months and lasts for several years.
On the other hand, patients with homogeneous emphysema or patients with alpha-1 antitrypsin deficiency have a less predictable outcome but should not be excluded per se, but selection has to be done particularly cautiously. It is very advisable to exclude all patients from LVRS with an exceedingly low functional reserve such as a diffusing capacity below 20% predicted or with pulmonary hypertension in combination with extreme parenchymal loss (vanished lungs) on CT. However, those patients with homogeneous emphysema who are severely impaired through respiratory mechanics shown clinically by their pathologic breathing pattern, as well as by the depressed diaphragm and distended ribs on chest radiography and hyperinflation on plethysmography (elevated TLC and RV), are potential candidates when sufficient gas-exchanging tissue (preoperative DLCO > 20%) is left behind. Additionally, cofactors which may potentially interfere with a smooth postoperative course such as previous recurrent infections, known extensive scarring of the lungs or previous surgery have to be taken into consideration in these patients since they are usually not profiting from LVRS over a longer period of time.
Emphysema Morphology
Emphysema is defined anatomically and characterised on CT by the presence of areas of low attenuation. In its severe form, it can easily be detected on a plain posteroanterior and lateral chest radiography. However, the most reliable method of obtaining information on the degree and distribution of emphysema is chest CT scanning. In addition, CT is even more sensitive and more specific than pulmonary function tests for the diagnosis of emphysema. Sanders et al. [14] reported that up to 69% of smokers with emphysema show normal pulmonary function tests.
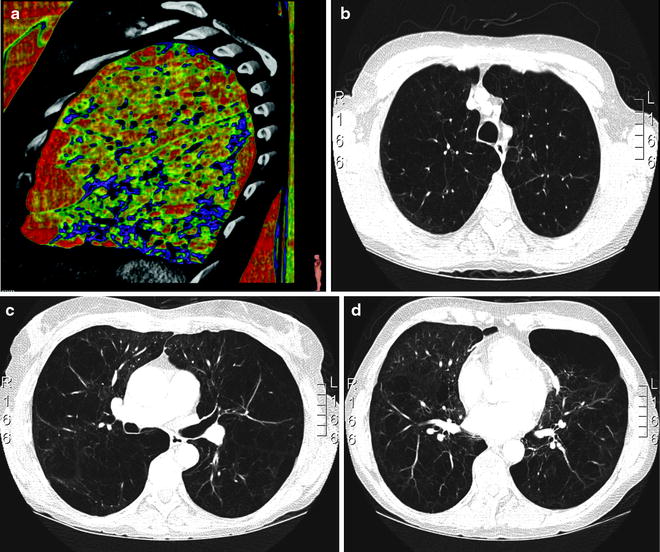
In our experience, chest CT plays a key role in the selection process for LVRS. Lung densitometry measurements (measuring volumes of zones with different densities) are very helpful for detecting the extent of emphysematous destruction and hence for planning the surgical procedure (Fig. 50.1). In general, areas of fully destroyed tissue are considered for resection, and the resected volume should leave approximately as much lung volume behind as the predicted total lung capacity is calculated. This concept is not validated scientifically by data but seems reasonable to us, and the definition of the lung volume to resect is especially important for patients with homogeneous disease, where target areas are not defined by its destruction. Different morphological grading systems have been developed to quantify the type, severity and distribution of emphysema as a help in identifying candidates for LVRS although no internationally accepted standardised radiological classification exists [15]. A specifically LVRS-oriented classification system based on CT findings was proposed by our group distinguishing between homogeneous, moderately heterogeneous and markedly heterogeneous emphysema distribution, and the predominance of the involved lobes was considered [16].
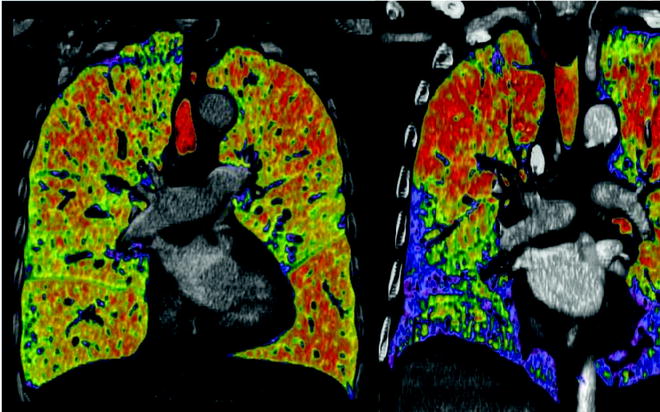

Fig. 50.1
Lung densitometry measurements left panel: non-heterogeneous distribution; right panel: heterogeneous distribution. Red marks lowest density lair
The following definitions were applied (Fig. 50.2). Markedly heterogeneous emphysema: a distinct regional difference in the severity of emphysema (i.e. decreased density, loss of vascular lung structure) is present in at least two adjacent lung segments of either lung. Intermediately heterogeneous emphysema: a distinct regional difference in severity of emphysema may be present maximally in the area of one or more than one but not in adjacent lung segments of either lung. Markedly heterogeneous: a distinct regional difference in the severity of emphysema is present in at least the area of two adjacent lung segments of either lung. This classification system is easy to apply, helps to select patients for LVRS and allows comparison of outcome. Quantitative perfusion scintigraphy is useful as additive method for confirmation of the target areas for resection.
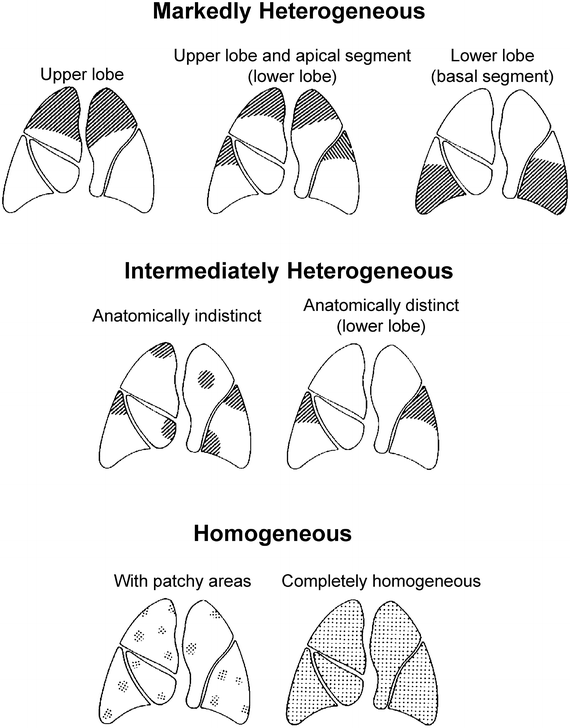

Fig. 50.2
(According to Weder et al. [16]): Classification system of emphysema: Three major types of emphysema distribution were defined: Markedly heterogeneous (upper panel), intermediately heterogeneous (middle panel) and homogeneous (lower panel). For heterogeneous emphysema types, the most affected areas were recorded as disease predominance in either upper lobe, upper lobe and apical segment of the lower lobe, or lower lobe Among homogeneous types of emphysema (lower panel), some showed multiple small zones of destruction throughout all lobes (patchy). In other emphysematous changes were evenly distributed throughout the entire lungs (homogeneous)
Lung Volume Reduction Surgery Techniques
LVRS is typically performed under general anaesthesia during one-lung ventilation via either median sternotomy, thoracotomy or VATS (video-assisted thoracoscopic surgery). Staple lines can be buttressed or not buttressed with bovine pericardium or synthetic reinforcement material in order to decrease the amount and duration of air leaks. LVRS is performed uni- or bilaterally. The target areas and the extent of resection differ between various types of emphysema. Staple lines should follow the parenchymal circumference in order to preserve the lung’s original shape in relation to the thoracic dimensions and to prevent cavities.
Median Sternotomy Versus VATS
Despite the NETT, the question about the role of median sternotomy or a thoracoscopic approach regarding safety and most efficacious approach for LVRS is still a matter of discussion, and the approach selected is according to the surgeon’s preference.
In 1996, Cooper’s group reported their results achieved by a bilateral LVRS through a median sternotomy on 150 patients. The 90-day mortality was 4%. The major complication was prolonged air leak. Kotloff et al.[17] reported on their experience with 120 patients undergoing bilateral LVRS, 80 by median sternotomy and 40 by VATS. The 30-day mortality is not significantly different (4.2% for median sternotomy and 2.5% for VATS); however, the total in-hospital mortality for the median sternotomy group was 13.8%, while it remained 2.5% for the VATS group. There was no significant difference in duration of air leaks or length of hospital stay between the two groups. Functional outcomes achieved with the two techniques were similar. In the NETT [18], 359 patients received lung volume reduction surgery by median sternotomy, and 152 patients by VATS. The 90-day mortality was 5.9% for median sternotomy and 4.6% for VATS. Both techniques had a similar outcome in terms of improved quality of life and functional outcome at 12 and 24 months. The complication rate and morbidities were low for both procedures and did not differ, but the VATS approach allowed earlier recovery at a lower cost than median sternotomy. According to reports and personal communications, most centres in Europe favour the VATS approach. We prefer a bilateral VATS approach as first choice and observed a 30 day mortality of 0.9% in the last 250 consecutively operated patients.
Unilateral Versus Bilateral LVRS
Several groups compared unilateral with bilateral LVRS. Not surprisingly, they found pulmonary function to be improved more following bilateral than unilateral surgery. However, there are conflicting data in regard to the overall duration of improvement when sequential unilateral surgery was done, but randomised studies were not performed addressing this question. The operative mortality was similar in both groups, whereas long-term survival has been reported to be longer in the bilateral group so that bilateral LVRS became the standard operative technique for patients with severe emphysema [19–21]. However, there is clearly a place for continued selective use of unilateral LVRS typically for patients who are not candidates for bilateral LVRS, in cases with predominantly unilateral disease, tumour or extensive pleural scarring on one side.
VATS Procedure
LVRS by VATS is performed under general anaesthesia with a placed double-lumen endotracheal tube to facilitate sequentially ventilation. A thoracic epidural is advisable to ensure adequate morphine-sparing analgesia so that postoperative pulmonary rehabilitation can proceed immediately after surgery. If the resection is planned on both upper lobes, a supine position is selected which allows an adequate access to both sides of the chest without changing position. On the other hand, the patient is placed in a lateral decubitus position, and changed sequentially when resection is planned on both lower lobes.
For upper-lobe LVRS and the patient is placed in supine, the trocar for the thoracoscope is introduced in the 7th intercostal space in the anterior axillary line whereas the first working incision is usually placed in the 5th intercostal space just lateral to the midclavicular line and the second working incision in the 6th intercostals space in the posterior axillary line. However, the exact position of the working incisions can be modified after visualising the intrathoracic anatomy once the thoracoscope has been placed. For lower-lobe resections and the patient in supine, the incisions are selected one intercostal space distally and more dorsally (midline for the thoracoscope and anterior and posterior axillary line for the lung forceps and staplers).
The lung is resected in areas that show the most severe emphysematous destruction on imaging studies (CT scan) corresponding with loss of perfusion on quantitative perfusion scan (heterogeneous type) [22]. This is typically in the upper lobes (approximately 30–50%) or the basal segments of the lower lobes. Some patients have a combination of upper-lobe (apical) and lower-lobe (apical segment) destruction. In those patients, approximately 20–30% of the upper lobe is resected, in combination with the apical segment of the lower lobe. In patients with homogenous emphysema, it is more difficult to define the amount and site of resection since clearly defined target areas are absent. In these cases, we preferentially choose the upper lobes for resection, and the amount of resection is the volume which is needed to reduce the total lung capacity to its predicted volume, usually approximately 40–50% of both upper lobes as discussed above. Since the resected lung volume cannot be exactly quantified during surgery, the question of the ideal volume of resection cannot be studied scientifically.
Once the resection is completed, the specimen removed and the haemostasis completed, a single 20–24 French chest tube is inserted with the tip oriented towards the apex of the lung, and in case of adhesions with the potential risk of bleeding, a 24-French chest tube is directed towards the diaphragm. The lung is thereafter re-expanded gently using room air with peak airway pressures not exceeding 20 cm H20. The chest tubes are connected to suction (−5 cm H20) or on water seal without suction in order to minimise trauma to the lung due to excessive negative intrapleural pressure (examples see Figs. 50.3, 50.4, 50.5, 50.6, 50.7, 50.8, 50.9, and 50.10).
Get Clinical Tree app for offline access