Surgical Treatment of Breast Carcinoma
11.1 Significance of Surgery in the Context of Multimodal Treatment of Breast Carcinoma
The surgical treatment of breast cancer has changed significantly in the last few decades. The reason for this is an improved understanding of the complexity of the disease, its biological behavior, and the impact on various therapeutic options (surgery, systemic therapy, radiation therapy). Each surgical treatment is integrated into a multidisciplinary adjuvant-therapy scheme (multimodal treatment) that must be individually determined for each patient. With improved early detection, breast cancer is increasingly detected in its preliminary stages, the treatment of which is fundamentally different from that of invasive carcinoma.
Preoperative determination of the histology using core needle or vacuum-assisted biopsies has also led to distinctly improved and better targeted surgical treatment. Preoperative knowledge of important tumor-biological information can simplify surgical planning significantly. To this end, the standardized histological assessment of the core needle biopsy with regard to its histological malignant potential is of great importance.
11.2 Types of Breast Carcinoma
11.2.1 Lesions of Uncertain Biological Potential (B3 Lesions)
Mammography screening is detecting more and more clinically occult lesions, the clinical significance of which still cannot be conclusively clarified. Lesions in this group include:
Flat epithelial hyperplasia.
Atypical ductal hyperplasia.
Lobular intraepithelial neoplasia (LIN).
Papillomas.
Radial scars.
Phyllodes tumors.
In addition to their individual risk of progression, these lesions also serve an “indicator” function (giving an indication of malignant events in their immediate vicinity). Moreover, they can indicate a general increased risk of disease (risk marker).
For a B3 lesion verified by core needle or vacuum-assisted biopsy, the intent of surgical intervention is primarily diagnostic. In about 20% of cases, there is an “upgrade” to a preinvasive or invasive lesion on the basis of the final surgical specimen. Due to the very diverse risks involved in B3 lesions, the indication for surgical intervention in each case should be determined in discussion by an interdisciplinary team including radiologist, surgeon, and pathologist.
11.2.2 Preinvasive Carcinoma (Ductal Carcinoma in Situ; B5a)
The morphology and biological risk potential of DCIS differs importantly from that of invasive carcinomas. In contrast to invasive carcinoma, DCIS is a locally circumscribed disease. The tumor cells grow inside the milk ducts without passing through the basement membrane. Thus, metastasis is essentially not possible in the case of pure DCIS. The risk of progression of DCIS to an invasive carcinoma is 14 to 52%. Surgery is the most important treatment modality for DCIS; it is significantly more effective than radiotherapy or systemic medical treatment. Even without adjuvant measures, mastectomy achieves a cure rate approaching 100%. Because of the lack of potential to metastasize, and the corresponding minimal threat to life, breast conservation has been adopted as standard therapy in cases in which the spread of the lesion is limited. This approach involves accepting a small risk of recurrence: this risk is diminished, but not entirely reversed, through the use of adjuvant radiation therapy. It should be noted that 50% of recurrences of DCIS have the appearance of invasive tumor, so that the quality of the primary surgery is extremely important.
11.2.3 Invasive Carcinoma (B5b)
Surgical excision of the primary tumor and regional lymph nodes constitutes an important pillar in the multimodal therapy concept of invasive breast carcinoma treatment. The extent of surgery can be significantly reduced by adjuvant therapy measures, such as systemic and radiation therapy.
In previous times the goal of breast carcinoma surgery was mechanical excision of all accessible tumor cells, both those that are clinically apparent and those that are occult, resulting in very radical surgery. This included resection of the entire breast (including the pectoral muscles, if required) and all accessible lymph nodes. Even for small tumors, a supplementary course of radiotherapy to the chest wall and regional lymph drainage areas was added to ensure local tumor destruction. This radical procedure was associated with high morbidity (▶ Fig. 11.1).

Fig. 11.1 Radical mastectomy. (a) Radical mastectomy (Rotter–Halsted) with excision of the breast, the pectoral major muscle, and the axillary lymph nodes. (b) Radical mastectomy (Rotter–Halsted) with additional irradiation. Formation of secondary lymphedema of the right arm.
In the second half of the 20th century, large randomized studies showed that surgical radicality did not affect overall survival. Thus the “extended radical mastectomy” (mastectomy including the pectoral muscles) offered no advantage when compared to the “modified radical mastectomy” (mastectomy preserving the pectoral muscles). Later, it was shown that breast-conserving therapy (BCT), with irradiation of the breast tissue that remained after surgery, had the same survival rate as complete excision of the breast (▶ Fig. 11.2).

Fig. 11.2 Comparison of surgical techniques and survival curves with the Halsted radical mastectomy. From pooled study data from the Milan Trials (I, II and II). (a) Overall survival rate of patients who have undergone various combinations of surgical and radiological treatments. (b) Cumulative incidence of local recurrence depending on the treatment used (all patients). Halsted: Halsted radical mastectomy. QU.A.D: quadrantectomy without radiation therapy. QU.A.RT: quadrantectomy with radiation therapy. T.A.RT: tumorectomy with radiation therapy.
The findings from these studies have led to an important strategic paradigm shift in the treatment of breast cancer. Invasive carcinoma of the breast was understood to be a systemic disease, the prognosis of which is determined by the biology of the individual tumor type and its potential for distant metastasis. It was much more the improvement of systemic medical treatment (hormonal and chemotherapy) and less the mechanical tumor destruction (surgery and radiation therapy) that became the focus of interest. This development was the foundation for our current treatment concept that surgery secures local tumor control whereas systemic therapy has the most important influence on prevention of distant metastases and thus on overall survival. Medical treatment also influences locoregional recurrence. Despite of the continuing reduction in extensiveness of surgery, the overall survival in breast carcinoma has been dramatically improved through the use of the multimodal treatment concept.
As well as controlling the tumor locally, surgical excision of the primary tumor and the lymph nodes provides important diagnostic and prognostic information. Both the tumor size (pT staging) as well as the nodal status (pN staging) are determined on the basis of the surgical specimen.
Note
For advanced local tumors or tumors with an unfavorable biology (triple-negative, HER2-positive), the treatment sequence of surgery first followed by systemic therapy is increasingly being reversed—surgery is preceded by medication treatment, most often chemotherapy (neoadjuvant therapy).
This procedure allows reduction of radical surgery and the increased use of breast-conserving therapy, and also this “in vivo chemosensitivity test” provides further important prognostic information.
11.3 Surgical Treatment of the Primary Lesion
11.3.1 Oncologic Aspects
Invasive Carcinoma
The fundamental principle for the treatment of primary invasive breast carcinoma is complete surgical resection with free margins (“in sano”). 19 This can be carried out either as a mastectomy or as breast-conserving surgery. In appropriate cases, breast-conserving therapy is comparable to mastectomy in terms of survival, and so breast-conserving surgery represents the standard procedure for surgical treatment of invasive carcinoma of the breast.
Take Home Points
General Basic Principle for Tumor Resection of Invasive Carcinoma
The fundamental principle for the treatment of nonadvanced breast carcinoma is resection of the tumor in sano.
Goal of Breast-conserving Therapy of Invasive Carcinoma
The goal of operative treatment is tumor excision. With reference to survival, breast-conserving therapy (BCT) with subsequent radiotherapy of the entire breast is comparable to modified radical mastectomy (MRM) alone.
Breast-conserving procedures are not possible in the following situations:
Multicentric carcinoma.
Inflammatory carcinoma.
Extensive microcalcifications.
If radiotherapy is not possible.
If surgical excision of the tumor in sano is not possible while still preserving the breast.
As part of BCT, it is obligatory to treat the remaining breast tissue with radiation therapy. With this, the local recurrence rate has been reduced from over 40% to the current rate of under 5%. Invasive foci of carcinoma are still commonly detectable microscopically at a distance of several centimeters from the primary lesion. A complete and reliable excision of all tumor cells is thus not clinically feasible and, notably, not verifiable. Even a resection “in sano” does not preclude microscopic tumor residues, but these can be controlled by means of the radiation therapy.
The optimal resection margin for invasive carcinoma in BCT has been intensively and controversially debated. Unfortunately, there are no available data from prospective studies concerning this question. A meta-analysis published in 2010, however, unambiguously demonstrated the impact of the free surgical margin on the recurrence rate. 13 Patients with free surgical margins displayed significantly lower recurrence than women with “narrow” or “affected” resection margins, though widening the resection to a healthy tissue margin of 2 to 5 mm did not lead to any further improvement in the results.
Therefore, according to current guidelines, a free surgical margin (“no ink on tumor”) is recommended. Widening the resection further can worsen the cosmetic result without reducing the recurrence rate.
Ductal Carcinoma in Situ
As opposed to invasive carcinoma, ductal carcinoma in situ (DCIS) is treated as a local disease rather than a systemic one. The quality of the surgery is therefore of particular importance. The goal of surgical treatment is complete resection of the entire affected tissue area.
DCIS is generally a unicentric lesion that spreads within a mammary duct system, rarely affecting multiple milk ducts. During their spread, the tumor cells can exhibit a discontinuous growth pattern. The “gaps” between the tumor parts can extend up to 1 cm. In contrast to the case with invasive carcinoma, therefore, a wide resection margin is very important here; a free resection margin per se does not guarantee a reliable surgical excision of the entire lesion, and adjuvant treatment modalities are less effective for DCIS than for invasive carcinoma.
As with invasive carcinoma, there are no prospective studies available on which to base the determination of an optimal resection margin. This is especially important because, for the majority of women who receive BCT, adjuvant radiotherapy is indicated. According to a meta-analysis by Dunne et al, 8 a resection margin of 2 mm appears to be adequate for these women. However, a subsequent meta-analysis by Wang et al suggests that even women who receive radiotherapy can benefit from a widened surgical margin of 1 cm. 31
Take Home Points
Goal of Surgery for Preinvasive Lesions
The resection margin is an important prognostic factor for DCIS. The tumor-free distance to the surgical margin should be at least 2 mm if postoperative irradiation is administered.
As a rule, DCIS is not clinically apparent. It is diagnosed by way of early detection mammography showing suspicious microcalcification formations or by linear-dendritic areas of increased enhancement on MRI. During surgery, the surgeon must make allowance for the fact that the histomorphological extent of the DCIS is usually greater than the area of microcalcification demonstrated on imaging.
Lesions of Uncertain Biological Potential (B3 Lesions)
Surgery for a B3 lesion has both diagnostic and therapeutic functions. After excision, approximately 20% of the changes turn out to be high-grade neoplasia (demonstrating the diagnostic function of the excision). B3 lesions carry their own risk of progression, so resection of them also has therapeutic intent. Adherence to a specific surgical margin is not mandatory for B3 lesions.
11.3.2 Technical Aspects
Resection
After prevention of local recurrence, an important goal in the surgical treatment of breast carcinoma is the preservation of an aesthetic body image. The quality of primary surgery can even influence the long-term surveillance of the breast positively by preventing postoperative seromas, extensive scarring, and fibrosis. To minimize the burden on the patient, the number of procedures required to achieve complete tumor resection should be kept as low as possible. According to currently available data, a re-resection is not mandatory in cases with close surgical margins of 1 mm or less. 19 The rate of second procedures can be minimized by intraoperative imaging (sonography or radiography of the specimen) and by confirmation of the macroscopically determined surgical margin by the pathologist. In doubtful cases, an intraoperative re-resection can be performed.
Note
Pretherapeutic determination of the surgical target volume is of great importance in assuring the complete closure of the tissue defect created by the surgery.
It is important to cover all defects to prevent hollows in the tissue, which could fill with seroma, in turn leading to widespread fibrosis and scar formation. These could hinder further imaging surveillance of the breast and adversely effect the cosmetic result. A preoperative interdisciplinary case conference including radiologist, surgeon, and pathologist, is necessary for optimal breast volume assessment—which in particular must include compilation of associated preoperative tumor elements.
Palpable lesions: For palpable lesions, the surgery can be performed using digital guidance. Intraoperative use of sonography is becoming increasingly established to ensure the reliable excision of the target volume, but also to spare healthy tissue as much as possible.
Nonpalpable lesions: Due to the increased use of early breast cancer detection methods, including mammographic screening, lesions are identified ever more frequently that are only detectable using imaging (sonography, mammography, MRI) and that exhibit no palpable counterpart. Image-guided marking is required for the surgical excision of such a nonpalpable lesion, and should be done using the simplest modality by which the lesion (mass lesion, areas of microcalcification, or non-space-occupying enhancement) is best visualized. The marking is accomplished using sonographic, mammographic, or MRI-guided wire marker placement. The tip of the wire should be placed as close as possible to the target lesion. In the case of extensive areas, such as extensive microcalcifications, it is a good idea to mark several surgically relevant corner points of the target volume to be excised.
An imaging examination of the surgical specimen (specimen sonography or mammography) must be carried out to ensure the reliable removal of the target area when surgically excising nonpalpable lesions.
An intraoperative frozen specimen of the surgical specimen should not be obtained for nonpalpable lesions because the technique of specimen preparation would cause loss of tissue. Intraoperative pathological evaluation is rarely necessary and is only indicated in the case of a palpable tumor. This is performed as a macroscopic evaluation of the surgical margin and merely represents an orienting initial examination to minimize the rate of follow-up resections.
Because the exact spread of a breast carcinoma (especially its accompanying, preinvasive intraductal tumor extensions) is frequently not identifiable on imaging, a secondary reexcision may be determined to be necessary after the complete processing of the surgical specimen. Due to the segmental spread of intraductal tumor parts, it is advisable to perform a resection following the orientation of the mammary duct segments. Using this technique, a more radical resection is pursued in the axis that has the greatest probability of containing occult tumor extensions than in glandular tissues that have a lower probability of residual tumor extension.
Unambiguous topographic marking of the specimen is essential in the evaluation of the surgical margins to enable a targeted follow-up resection in case of narrow or insufficient excisional margins. For this, the specimen is marked with sutures. Ideally, it is also pinned onto a template to document the relationship of the resection margins to the remaining mammary glands in a reproducible way (▶ Fig. 11.3).

Fig. 11.3 Perioperative and radiographic processing of the surgical specimen. (a) Lateral view of the template. (b) Surgical specimen attached to the template. (c) Topographical identification markings for a surgical specimen. (d) Topographical identification markings for a follow-up resection. (e) Specimen radiograph while maintaining the topographical orientation. (f) Specimen radiograph (lateral image).
Targeted irradiation of the tumor bed (radiation boost), in addition to the adjuvant irradiation of the entire breast, has been shown to significantly reduce the local recurrence rate. The tumor bed or resection margins should be marked intraoperatively with titanium clips to enable the radiation therapists to plan for the radiation boost to the area as precisely as possible, and to minimize the target volume (▶ Fig. 11.4).

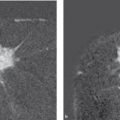
Fig. 11.4 Marking the tumor with clips. Mammographic visualization of two radiopaque clips in the area of the resection.
Defect Reconstruction–Oncoplastic Surgery
Along with an adequate surgical resection, the preservation or restoration of an aesthetic breast shape is an important target criterion of surgical treatment. To satisfy breast-conserving concepts, every tissue defect must be systematically reconstructed so as to avoid retractions or collections of serum with resultant contractures. Depending on the size of the defect, a variety of different techniques come into play, all of which are included in the term “oncoplastic surgery.” This is understood combine an oncologically oriented resection with the immediate reconstruction of the defect. To this end, a multitude of operative techniques are employed.
Glandular flap reconstruction: Smaller tissue defects can be closed by moving local (autologous) glandular tissue. A variety of techniques are used in a procedure that ranges from simple adaptation of the resection margins to a wide-ranging mobilization of the entire mammary gland and the preparation of an extensive glandular rotation flap.
Tumor-adapted mastopexy: In the tumor-adapted mastopexy, the skin envelope surrounding the breast is adapted to fit the smaller breast volume. This method may be required if a defect cannot be covered with autologous tissue. It can also be used if it is necessary excise the overlying skin because of its proximity to the tumor. This usually leads to distortion of the breast and/or the nipple-areola complex. An unfavorable result can be avoided only by reshaping the skin envelope. Depending on the location of the tumor, various incisional patterns can be used. Recentering of the nipple–areola complex is usually required when a mastopexy is performed (▶ Fig. 11.5).
Tumor-adapted reduction mammoplasty: In this method, further breast tissue is excised in addition to the target volume removed for oncologic reasons. In the case of large tumors with unfavorable locations, a reduction mammoplasty with wide excision margins can result in a good cosmetic result. And the effect of future radiotherapy can be improved in very voluminous breasts. The various techniques used when performing a tumor-adapted reduction mammoplasty differ in the pedicle-flap techniques used to reposition the nipple. As a rule, when a tumor-adapted reduction mammoplasty is done, an equalizing procedure on the other breast will have to be performed as well (▶ Fig. 11.6).
Defect coverage using transfer of distant tissue: When the relationship between the breast and the required resection volume is unfavorable, the resected glandular tissue (and skin, if applicable) can also be covered by the transfer of distant tissue. This comes into play if the skin envelope, or the entire breast itself, is insufficient to allow a tumor-adapted mastopexy or a breast reduction because of the large resection volume or because a breast is primarily very small. In breast-conserving surgery, the latissimus dorsi muscle can be used as a myocutaneous flap. In this procedure, a flap of skin and fat is transferred from the back to the chest wall. To maintain tissue perfusion, the latissimus dorsi muscle, serving as a “guide rail” for the thoracodorsal vascular bundle, is resected along with the overlying skin and adipose tissue. Only the narrow, superior insertion of the muscle remains intact in its original position. On this pedicle, the autologous tissue from the back is rotated to the chest wall (reconstruction using a myocutaneous pedicle flap; ▶ Fig. 11.7)

Fig. 11.5 Mastopexy. Tumor-adapted mastopexy. Reduction and restructuring of the skin envelope to preserve an aesthetic result (e.g., when the tumor is located close to the skin). (a) Reference lines for the surgical incision. (b) Results of the surgery.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree