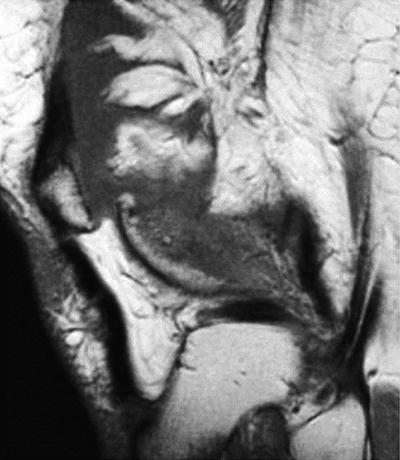
Fig. 10.1
Sagittal proton-density magnetic resonance image without fat saturation illustrating pigmented villonodular synovitis with bony erosion of medial tibial plateau
MR imaging is the method of choice for diagnosis, surgical planning, and evaluation of PVNS [14, 15, 32, 33]. Characteristic MR imaging features of PVNS enable a diagnosis to be made in 83–95 % of cases [24, 32].
In localized or focal PVNS, the classic MR image appearance is a solitary ovoid lesion with low signal intensity on both T1- and T2-weighted imaging. The most common location is the infrapatellar fat pad but areas may also include the suprapatellar pouch, intercondylar notch, and lateral synovial gutter [15, 22, 32].
In diffuse PVNS, MR images of the knee reveal synovial thickening, hypertrophy, and irregularity most commonly in the regions of the suprapatellar bursa and posterior joint recess. The most reliable diagnostic feature is the deposition of hemosiderin-laden macrophages viewed on T1- and T2-weighted images and on echo gradient imaging. As seen in Fig. 10.2, the synovial thickening can produce diffuse low-signal-intensity masses. On gradient echo imaging, these demonstrate a characteristic “blooming” artifact of hemosiderin deposition due to the paramagnetic effects of the iron [15, 32]. Diffuse PVNS exhibits an extensive pattern of hemosiderin deposition within the synovium resulting in frond-like low signal irregularities within the joint capsule. Enhancement is seen on T1-weighted images after IV gadolinium injection. Diffuse disease can extend beyond the joint capsule, and common extra-articular sites of involvement around the knee include the semimembranosus bursa, the popliteus tendon sheath, and previous arthroscopy portals.
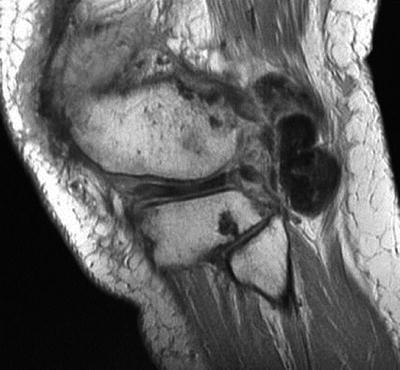

Fig. 10.2
Sagittal proton-density magnetic resonance image without fat saturation reveals low-signal mass extending from the posterior joint space of the knee
Treatment
Localized PVNS
Lesions can be sessile or pedunculated and are often limited to a single location, most commonly intra-articular in location. Nonoperative management may include physical therapy and intra-articular corticosteroid injections; however, these are of limited utility in transiently improving mechanical symptoms and offer no benefit for definitive treatment.
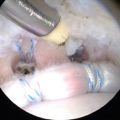
Nodular excision with partial synovectomy (Fig. 10.3) can often be performed arthroscopically with relief of symptoms and 0 % recurrence rate at short- and mid-term follow-up [22, 27, 34–37]. Open local excision may be performed for extra-articular involvement and for inaccessible intra-articular disease such as the posterior knee or fixed synovial disease [17].
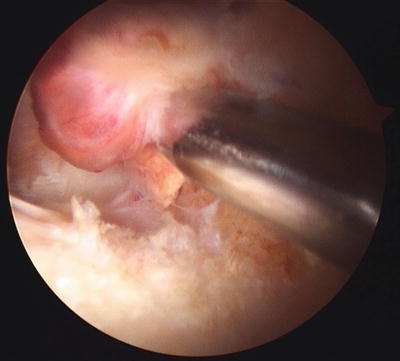

Fig. 10.3
Arthroscopic view of focal, localized pigmented villonodular synovitis lesion
Diffuse PVNS
Although considered to be a benign disease, diffuse PVNS can be locally aggressive and cause joint damage. Like local PVNS, nonoperative management may include physical therapy and corticosteroid injections to temporarily improve localized pain and swelling; however, this will not treat the underlying disease pathology. External beam radiation therapy has been described as primary treatment for unresectable lesions or in nonoperative candidates with recurrence rates of 7–67 % [38–40].
Surgical treatment for diffuse PVNS remains controversial and depends on the extent of the disease. Some authors advocate arthroscopic excision with complete synovectomy as the treatment of choice [34, 35, 41]. Arthroscopic treatment offers the patient a faster postoperative recovery to baseline function and decreased likelihood of stiffness [17, 27]. However, incomplete excision is not uncommon and often leads to recurrence. Recurrence rates approach 25 % for intra-articular involvement and 50 % for extra-articular disease, depending on the extent of the primary excision [20, 42]. For this reason, many surgeons prefer open arthrotomy excision with complete synovectomy, particularly for large-volume diffuse PVNS [17, 26, 42–44]. An open, posterior approach may also be necessary to excise extra-articular extension along tendons within the popliteal fossa. Adjuvant radiosynovectomy with intra-articular injection of yttrium-90 can be used after synovectomy to decrease recurrence rates of intra-articular lesions; however, there is a paucity of evidence and limited case series [45–47].
Case 2: Synovial Chondromatosis
A 46-year-old male presents with insidious-onset pain, swelling, crepitus, and decreased range of motion in his right knee for the past 2 years. He denies any history of trauma. On physical examination, an effusion is present and there is a palpable suprapatellar mass.
Introduction
Primary synovial chondromatosis is a benign disease first described by Leannac in 1813 but not formally named until 1958 by Jaffe [48–51]. It is rare and has an incidence of 1 per 100,000 people [52, 53]. It is two to four times more likely to develop in men and most commonly presents in the third to fifth decades of life [48, 49, 54]. Although clonal abnormalities and rare malignant changes have been reported in synovial chondromatosis, the disease process is generally categorized as a metaplastic rather than neoplastic disease of synovial cells [55–59]. It is characterized by chondroid metaplasia of synovial mesenchymal cells with resultant formation of lobulated, pedunculated intra-articular chondral bodies. These often shed into the joint, which may then ossify (osteochondromatosis). In 1977, Milgram described a clinical progression of this disease process by delineating its course into three phases: (1) active intrasynovial disease only with no loose bodies; (2) transitional lesions with both active, intrasynovial proliferation and free loose bodies; and (3) multiple, free osteochondral bodies with no demonstrable intrasynovial disease [60].
Secondary synovial chondromatosis is associated with mechanical or arthritic joint abnormalities, which result in the formation of loose, intra-articular chondral bodies [61].
Clinical Presentation
Synovial chondromatosis is classically a monoarticular disease, although polyarticular involvement has been described in up to 5 % of cases [53, 62]. The knee is the most commonly involved joint (50–65 %), followed by the hip, elbow, and shoulder [49, 53, 63]. Bilateral knee involvement has been reported in up to 10 % of cases; however, most cases are likely a representation of secondary synovial chondromatosis [54]. Primary synovial chondromatosis is most commonly diffuse, involving the entire synovium of the affected joint; however, localized disease has been described [64, 65].
The most common sites of involvement within the knee are the suprapatellar pouch, infrapatellar fat pad, and the anterior interval between the ACL and the infrapatellar fat pad [66, 67]. Less commonly, the posterior compartment (posterior to the PCL) may be involved [65, 66, 68, 69].
Patients present with a subacute onset of pain (85–100 %), swelling (40–58 %), and limited range of motion (35–55 %). Patients seldom recall an antecedent trauma to the knee and have no apparent systemic signs of infection or illness. On physical exam, patients often have an effusion, tenderness to palpation, articular crepitus (20–33 %), locking (5–10 %), palpable nodules, or a distinct mass (5–20 %) [54, 64, 70–72].
Histopathology
The gross appearance of synovial chondromatosis consists of hyperplastic synovium overlying white, nodular projections of hyaline cartilage diffusely scattered across the entire joint surface [49, 54, 73–75]. This often gives the synovium a “cobblestone” appearance. The nodules may detach from the synovium, thus creating free chondral bodies within the affected joint. The number of nodules can range from a few to thousands, depending on the stage of the disease. They vary in size from a few millimeters to a few centimeters in diameter [49, 54]. As the nodules increase in size, the central zone can undergo calcification and, rarely, endochondral ossification. Multiple detached nodules may coalesce to form a large mass termed giant synovial chondromas, though this is rare [54, 76].
Imaging
As seen in Fig. 10.4, conventional roentgenograms reveal intra-articular ossified bodies in 70–95 % of cases of primary synovial chondromatosis. Characteristically, they are innumerable, similar in size and shape, and evenly dispersed throughout the synovial lining of the affected joint [48, 49, 54, 73, 75, 77]. Periarticular bony erosions are common in more constrained joints such as the wrist, elbow, and hip; however, these are less likely in the knee and shoulder [78].
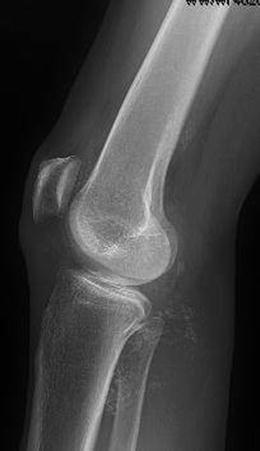

Fig. 10.4
Lateral radiograph of the knee, revealing multiple ossified bodies in the posterior compartment
Computed tomography (CT) imaging of the knee can be useful in differentiating primary synovial chondromatosis from other causes of soft tissue lesions, particularly when conventional roentgenograms are equivocal. Hyaline cartilage has a high water content and therefore low attenuation on CT imaging, which can be appreciated in the nonmineralized synovial thickening caused by synovial chondromatosis. Furthermore, the majority of nodules caused by synovial chondromatosis contain central and peripheral ossification which CT imaging delineates as a “ring-and-arc” pattern of mineralization or a target appearance [53, 54]. However, purely chondral lesions are better defined by magnetic resonance imaging.
MR imaging of the knee provides the optimal modality to aid in the diagnosis and treatment plan for primary synovial chondromatosis. Due to the heterogenous makeup of calcification and ossification of the nodules, variable signal characteristics are identified, as illustrated in Figs. 10.5 and 10.6 [54, 76, 79–81]. The most common pattern (77 %) demonstrates low/intermediate signal intensity on T1-weighted sequences and high signal intensity on T2-weighted sequences. This correlates clinically with the Milgram phase 2 lesions, as described above [54, 76].
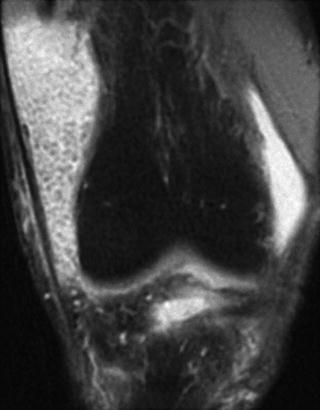
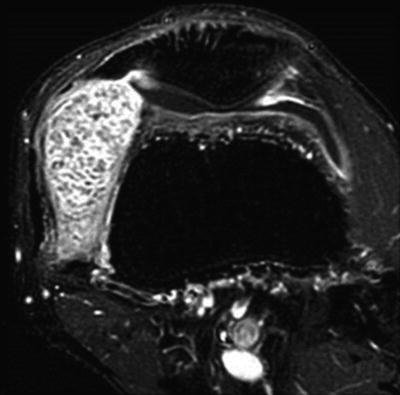

Fig. 10.5
Coronal fat-saturated T2-weighted magnetic resonance image revealing numerous low-signal osteochondral bodies

Fig. 10.6
Axial fat-saturated T1-weighted magnetic resonance image post IV gadolinium demonstrates multiple low-signal osteochondral bodies within the enhancing synovium
Treatment
Patients presenting with recurrent pain, swelling, and mechanical symptoms refractory to nonoperative management are candidates for surgical intervention. Primary synovial chondromatosis is generally a benign and self-limiting disease; however, it may be progressive and complicated by osteoarthritis. On rare occasions, synovial chondrosarcoma can arise in the setting of synovial chondromatosis (5 % incidence) [54, 75, 82]. Therefore, the treatment of choice is surgical excision of the osteochondral nodules with or without partial or complete synovectomy, depending on the extent of the disease. Surgical excision can be performed by open or arthroscopic approach, and the technique should be selected based upon ability to safely access and thoroughly remove all the diseased synovium and nodules.
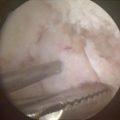
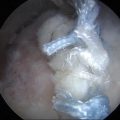
Arthroscopic excision offers the surgeon better visualization of the intra-articular synovium and decreased pain, stiffness, and postoperative rehabilitation course for the patient [65, 69, 83]. The need for partial or complete synovectomy for treatment of primary synovial chondromatosis remains controversial. Milgram recommended treating patients with phase 1 primary synovial chondromatosis (active intrasynovial disease only with no loose bodies) with synovectomy. For phase 2 (transitional form with active intrasynovial disease and chondral bodies), he recommended synovectomy with chondral body retrieval. Lastly, for phase 3 (late inactive intrasynovial disease with chondral bodies but no synovial abnormality), he recommended chondral body retrieval alone [60]. With this treatment regimen, Milgram observed recurrence rates of 12.5 %, 10 %, and 0 % for phases 1, 2, and 3, respectively [54, 60]. The recurrence rate for larger series ranges from 3 to 23 % and recurrence is most likely related to incomplete primary excision [63, 64, 70]. Ogilvie-Harris and Saleh reported nearly a 60 % recurrence rate when treating patients with loose chondral body retrieval alone [84]. Therefore, arthroscopic retrieval of chondral loose bodies and complete synovectomy is the treatment of choice for primary synovial chondromatosis. This was performed in our patient, as seen in Figs. 10.7 and 10.8.
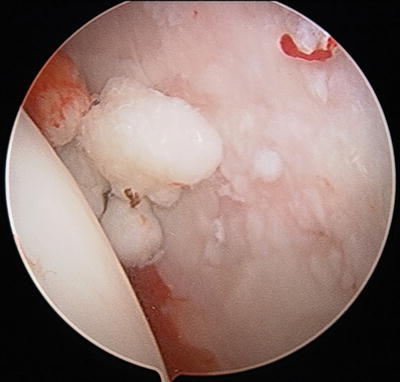
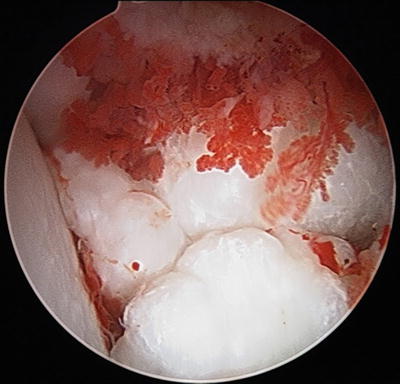

Fig. 10.7
Arthroscopic view of numerous osteochondral bodies embedded within the synovium

Fig. 10.8
Arthroscopic view of multiple osteochondral bodies and inflamed synovium
Case 3: Synovial Hemangioma
An otherwise healthy 13-year-old female with no history of trauma presents with recurrent effusions and decreased range of motion in her right knee.
Introduction
Hemangiomas and hemangiohamartomas are types of rare, benign vascular tumors that may affect the musculoskeletal system. Hemangiohamartomas, more commonly known as arteriovenous malformations, are differentiated from hemangiomas in that they contain fat, connective tissue, and peripheral nerve structures [29]. Hemangiomas, however, are composed primarily of closely packed thin-walled capillaries. Depending on their location in relation to the joint, hemangiomas may be described as juxta-articular (outside the joint capsule but in relation to it), intra-articular, extra-articular, or intermediate (both intra- and extra-articular) [85–87]. Both the intra-articular and intermediate types generally involve the synovial membrane. Synovial hemangiomas were first described by Bouchut in 1856 and further categorized as being either localized or diffuse in character by Bennett and Cobey in 1939 [88, 89]. They are exceedingly rare, with approximately 200 reported cases [90, 91]. Most commonly, they occur in children and adolescents with the average age of onset of 10.9 years in females and 12.5 years in males [92, 93]. Synovial hemangiomas are slightly more common in females (53 %) [91, 94, 95].
Clinical Presentation
Synovial hemangiomas have been reported in the wrist, ankle, and elbow, but they most commonly involve the knee [87, 91, 92, 96, 97]. The tumor may be localized and well circumscribed or diffuse in character. Patients often present with an insidious onset of knee pain and recurrent effusions with associated limitation in range of motion. Atrophy of the quadriceps muscle is common in patients with a synovial hemangioma [87, 95, 98]. Patients with localized and pedunculated synovial hemangiomas may have a palpable soft tissue mass and often experience mechanical symptoms. Diffuse involvement can lead to more pronounced synovial venous congestion and hemorrhagic synovitis. Patients with both localized and diffuse subtypes often have a diagnostic delay, because of the rarity of synovial hemangiomas [99–102]. This delay leads to many patients having a history of spontaneous swelling and recurrent hemarthrosis in the absence of coagulopathy. In up to 40 % of cases, the patient may present with overlying cutaneous hemangiomas and venous congestion of the knee [91, 93, 103, 104].
Histopathology
Synovial hemangiomas can be classified as cavernous, capillary, mixed, or arteriovenous [91, 105]. Most commonly, they exhibit features of cavernous hemangiomas with lobulated proliferation of capillary-sized vascular channels. Inflammatory cells and hemosiderin-laden macrophages are frequently identified, thus resembling pigmented villonodular synovitis. However, the existence of dilated, thin-walled capillaries with cavernous appearance differentiates PVNS from synovial hemangiomas [91, 97].
Imaging
Conventional roentgenograms may reveal the presence of an effusion, soft tissue mass, phleboliths, advanced maturation of the epiphysis, or periosteal reaction [95, 100, 103, 106]. Frequently, however, radiographs show no abnormalities and further imaging is necessary. CT imaging can aid in the diagnosis to rule out bony abnormality; however, the demarcation between soft tissue mass and muscle is limited.
MR imaging does not use ionizing radiation and provides better soft tissue differentiation to demonstrate the extent of involvement of the synovial hemangioma [105, 107–109]. In conventional MR imaging without contrast, the lesions are lobulated with well-defined margins and exhibit homogenous, low-to-iso-signal intensity on T1-weighted imaging and heterogeneous, high signal intensity on T2-weighted imaging, as seen in Figs. 10.9 and 10.10 [90, 100, 103, 106, 110, 111]. The use of gadolinium contrast has been advocated by some authors to differentiate synovial hemangiomas from ganglion cysts, cystic synovial hyperplasia, synovial sarcoma, leiomyoma, and synovial chondromatosis [90]. With Gd-enhancement, synovial hemangiomas exhibit heterogeneous enhancement (Fig. 10.11). This has been useful to differentiate synovial hemangiomas from other intra-articular synovial lesions with the exception of synovial sarcomas which can display similar characteristics. However, these malignant lesions most commonly arise extra-articularly [90, 112]. MR imaging allows for accurate preoperative assessment in the classification of the lesion, thus guiding a definitive treatment strategy.

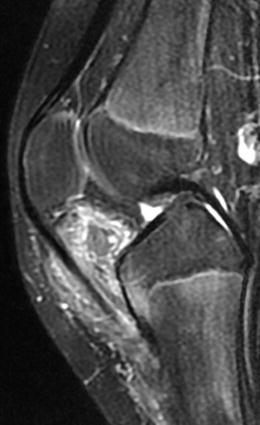
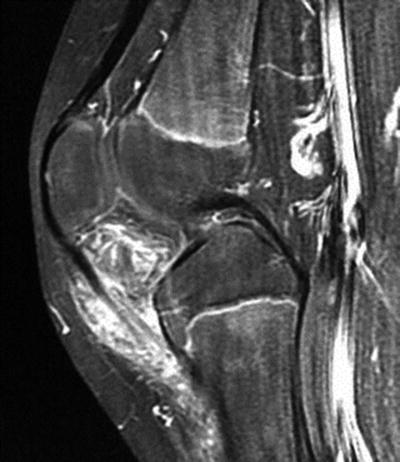

Fig. 10.9
Sagittal T1-weighted magnetic resonance image revealing infrapatellar lesion with internal fat signal

Fig. 10.10
Sagittal T2-weighted magnetic resonance image revealing infrapatellar lesion with internal high T2 signal and suggestion of peripheral vessels

Fig. 10.11
Sagittal fat-saturated T1-weighted magnetic resonance image post IV gadolinium enhancement demonstrates discrete vessels and strong enhancement consistent with synovial hemangioma
Treatment
Aside from pain, mechanical symptoms, and decreased range of motion, synovial hemangiomas frequently cause recurrent hemarthrosis, which can lead to early destruction of the articular surface of the knee. Therefore, several treatment options have been described for synovial hemangiomas including embolization and open or arthroscopic surgical excision with and without partial synovectomy. Like other synovial disorders, the treatment of choice depends on the classification and extent of involvement.
Localized synovial hemangiomas can often be treated successfully with arthroscopic surgical excision, especially when the lesion is pedunculated and well circumscribed [91, 105, 113, 114]. Frequently, localized lesions will have a single vascular supply that can be managed arthroscopically with minimal bleeding.
Diffusely involved synovial hemangiomas in a single compartment of the knee are better treated with open arthrotomy, surgical excision, and partial synovectomy [95, 98, 115, 116]. They often have multiple feeding vessels; therefore, preoperative angiography with embolization may improve surgical results and decrease postoperative bleeding [91, 99, 101]. However, in diffuse synovial hemangiomas that involve multiple compartments of the knee, nonoperative management with repeat imaging to evaluate progression has been encouraged [91, 98].
Case 4: Lipoma Arborescens
A 45-year-old male with diabetes mellitus presents with complaints of mechanical symptoms in his right knee and a palpable, tender mass over the superior aspect of his patella.
Introduction
Lipoma arborescens (diffuse articular lipomatosis) is a rare, benign intra-articular disease affecting the synovial membrane. First described by in 1957 by Arzimanoglu, it is characterized as a diffuse substitution of the joint’s subsynovial layer by mature adipocytes, resulting in the formation of villous projections [117–125]. Numerous villous lipomatous synovial proliferations differentiate lipoma arborescens from an intra-articular lipoma [120, 121, 126]. The etiology of lipoma arborescens remains unclear; however, developmental, traumatic, inflammatory, and neoplastic origins have been postulated [118, 123, 124]. Since its first description, there have been approximately 75 case reports presented in the literature [127]. There is a slight male preponderance (56–70 %) and the age range of disease onset is 10–90 years with a mean age of 37 years [127–129]. Associated conditions include osteoarthritis, rheumatoid arthritis, psoriatic arthritis, gout, joint trauma, and diabetes mellitus [118, 128, 130–133].
Clinical Presentation
Lipoma arborescens is a rare disorder that can affect the hip, shoulder, wrist, elbow, and ankle, but it most commonly affects the knee [117, 119, 124, 128, 129, 134]. Most cases are monoarticular; however, bilateral knee involvement has been described in up to 16 % of cases [127, 129]. The suprapatellar pouch is invariably involved. Other common sites include the condylar gutters and the premeniscal regions [117]. Patients with lipoma arborescens typically present with painless swelling of the knee, which leads to progressive restriction of range of motion. They often have intermittent exacerbations with resultant mechanical symptoms, as the villous projections can become trapped within the joint resulting in catching and locking [124]. Laboratory testing (ESR, CRP, WBC, uric acid, rheumatoid factor, HLA-B27) in patients with lipoma arborescens is normal [123, 129, 135]. Joint fluid aspiration reveals a serious appearance, is negative for cells and crystals, and is sterile on culture [124].
Histopathology
Macroscopically, lipoma arborescens is a fatty tissue mass with frond-like appearance. The finger-shaped synovial projections are numerous with broad bases [123, 127, 129, 136, 137]. Histologically, the disease is characterized by diffuse replacement of the subsynovial layer with mature adipocytes. Focal infiltration of perivascular mononuclear inflammatory cells is often appreciated [118, 119, 129].
Imaging
Conventional roentgenogram is of limited utility in the diagnosis of lipoma arborescens but may indicate the presence of a soft tissue mass and help to rule out bony involvement. CT imaging often demonstrates a soft tissue synovial mass and the characteristic synovial fronds may be outlined by an adjacent effusion. Low attenuation measurements are consistent with fat, and there is little or no enhancement after contrast administration [123, 130, 135, 138].
MR imaging is the modality of choice when evaluating a patient for lipoma arborescens. MR imaging reveals villous synovial thickening with fatty frond-like projections, usually with an effusion. The uniform fat signal and lack of hemosiderin aid diagnosis and differentiation from other lesions. (Fig. 10.12) [117, 133, 134, 139, 140]. Associated findings on MR imaging include chondral degenerative changes (87 %) and meniscal tears (72 %) [141]. Similar to that of subcutaneous fat, lipoma arborescens has high signal intensity on T1 (Fig. 10.13) and low signal on fat-saturated and STIR sequences (Fig. 10.14) [127, 141].