Key Points
- •
A classic imaging feature of systemic lupus erythematosus (SLE) is deforming arthritis without erosion.
- •
Avascular necrosis may be present in asymptomatic patients with SLE.
- •
Antiphospholipid syndrome may be a primary disorder or be associated with other diseases, especially SLE. Imaging reveals thrombosis of affected vessels and its sequela.
- •
Kawasaki disease presents special imaging considerations because it affects children and long-term follow-up is necessary. Echocardiography is a standard evaluation technique for this disorder. Magnetic resonance imaging (MRI) allows both the coronary artery lumen and wall to be evaluated without ionizing radiation and may be a useful technique. Computed tomography (CT) arteriography is useful but radiation exposure can be similar to that of coronary artery catheterization.
- •
Takayasu’s arteritis affects the aorta, its proximal branches and pulmonary arteries but not muscular arteries. Because inflammation of the vessel wall is a characteristic early feature, MRI, CT, and positron emission tomography CT may be useful. Later disease causes vessel stenoses or occlusions.
- •
Giant cell arteritis can be diagnosed by arterial biopsy. Recently ultrasound findings have been described that may provide an alternative to biopsy.
SYSTEMIC LUPUS ERYTHEMATOSUS
Definition and Criteria for Diagnosis
Systemic lupus erythematosus (SLE) is an autoimmune disorder that results in inflammation, deposition of immune complexes, vasculitis, and vasculopathy. It affects about 40 individuals per 100,000 population in North America, and more than 80% of cases occur in women of childbearing age. African-Americans and Hispanics are at increased risk of being affected.
The 1982 American College of Rheumatology (ACR) revised criteria for the classification of SLE allow the diagnosis to be made with 96% sensitivity and 96% specificity. Four or more of the 11 described criteria needed to be present to establish the diagnosis with this accuracy. These criteria have been further revised ( Table 22-1 ).

The diagnosis of SLE can be made with 96% sensitivity and 96% specificity using the revised ACR criteria.
Antinuclear antibodies are present in more than 95% of patients (although these may also be present in low titers in other disorders). Antibodies to native or double-stranded DNA and to Sm (anti-Smith antibody) are more specific for the diagnosis of SLE. Almost one third of SLE patients also have antiphospholipid antibody, which may cause thromboembolic complications including stroke, portal vein thrombosis, thrombophlebitis and pulmonary embolism, and repeated midtrimester abortions (see Antiphospholipid Syndrome, below).
Almost one third of patients with SLE have antiphospholipid antibody, which may cause thromboembolic complications.
Musculoskeletal Manifestations
Musculoskeletal manifestations of SLE include arthritis, avascular necrosis, soft tissue calcification, changes in the terminal tufts of the fingers, and tendon rupture.
Arthritis
Joint involvement is one of the earliest and most common manifestations of SLE. Thus it may precede other features of the disease. A higher incidence of deforming arthritis has been found with increased disease duration, a positive rheumatoid factor, and antibodies to U1 RNP. Patients with deforming arthritis have a higher frequency of sicca symptoms and lower incidence of facial erythema and photosensitivity. Longer disease duration and late onset disease have been noted in patients with radiologic abnormalities of the hands and feet.
Approximately 90% of patients with SLE have joint manifestations.
A symmetric polyarthritis that most often involves the hands and knees is typical. Arthritis is typically out of proportion to clinically evident synovitis and is episodic, polyoligoarticular, and migratory. The presence of a nonerosive arthritis involving two or more joints is, in fact, one of the clinical criteria used for establishing the diagnosis and is 86% sensitive and 37% specific. Effusions are usually not as large as in rheumatoid arthritis (RA) and not as inflammatory. Labowitz and Schumacher described the synovial fluid as clear to slightly turbid with good viscosity and fewer than 3000/mm 3 white blood cells with mononuclear cells usually predominating.
Radiography
Investigation of hand radiographs in 59 patients with SLE has shown radiographic abnormalities in 34 patients, although the most common features were nonspecific periarticular osteopenia or soft tissue swelling. Erosion is a less common finding in SLE than in RA owing to a lack of aggressive pannus on histologic examination in SLE.
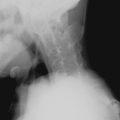
Deformity without Erosion
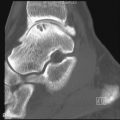
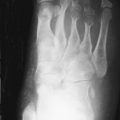
Deforming arthritis without erosion is a classic feature of SLE and is seen in up to 35% of patients ( Figure 22-1 ). This pattern is similar to that seen in postrheumatic fever arthritis (termed Jaccoud’s arthropathy ) but is unlike that seen in RA, in which erosion is a characteristic feature. The deformities that occur in lupus are not due to destructive synovitis. Instead they are thought to be a consequence of low-grade inflammation of the synovial membrane and capsule resulting in ligamentous laxity and muscular imbalance.




Deforming arthritis without erosion is a classic feature of SLE.
Weissman et al. found deformity of the hands to be most common at the proximal interphalangeal joints. Thumb interphalangeal hyperextension and swan neck deformity, ulnar deviation, and metacarpophalangeal (MCP) subluxations of the fingers were noted. Ulnar deviation is the earliest of these features. Thumb deformities begin as passively correctable flexion deformity at the MCP joint with hyperextension at the interphalangeal joint ( Figure 22-2 ). Later, if the carpometacarpal joint becomes unstable, and subluxation or dislocation and adduction of the thumb metacarpal occur, flexion develops at the intraphalangeal joint with hyperextension at the MCP joint. This is important because treatment will require stabilization of the carpometacarpal joint before other joints are addressed.


Spronk et al. developed an index to describe the severity of these deformities ( Table 22-2 ). Individuals with a score of more than 5 were classified as having Jaccoud’s arthropathy. In addition to finger deformities, scapholunate dissociation and ulnar translocation of the carpals may be seen. Changes in the feet consist of hallux valgus and subluxation of the metatarsophalangeal joints.
| Jaccoud’s Index | ||
|---|---|---|
| Number of Affected Fingers | Points Assigned | |
| Ulnar drift (> 20 degrees) | 1–4 | 2 |
| 5–8 | 3 | |
| “Swan neck” deformities | 1–4 | 2 |
| 5–8 | 3 | |
| Limited MCP joint extension | 1–4 | 1 |
| 5–8 | 2 | |
| Boutonniere deformities | 1–4 | 2 |
| 5–8 | 3 | |
| Z deformity | 1 | 2 |
| 2 | 3 | |
The absence of erosive disease after 2 years may suggest the diagnosis of SLE over RA.
van Vugt et al. reviewed 176 patients with SLE and found a deforming arthropathy (diagnosed as any deviation of a metacarpal/finger axis) in the hands of 17 patients. Cases of deforming arthropathy were subclassified as follows:
- 1
An erosive form (“rhupus”) demonstrating features simulating RA. This was identified in 3 of 17 patients.
- 2
Jaccoud’s arthropathy (“lupus hand”) based on the index described by Spronk was seen in 8 of 17 patients. Coexistence of Jaccoud’s arthropathy and the antiphospholipid syndrome (APS) was noted.
- 3
Mild deforming arthropathy was seen in 6 of 17 patients.
Erosive Arthritis
Although not the classical picture of SLE, approximately 1% to 2% of patients with the disorder develop an erosive arthritis resembling RA (termed rhupus ) . This may be due to the coexistence of the diseases or may represent a subset of lupus patients.
Bywaters described hook deformities at the MCP joints. These have the radiographic appearance of corticated erosions, and no active pannus is seen in these areas on pathologic examination. As in RA, erosive changes may also occur due to remodeling (compressive erosion) or due to chronic changes from adjacent inflamed tendons. Reilly et al. found ulnar styloid erosions (postulated to be due to adjacent tenosynovitis) in 28% of 51 cases of SLE.
Richter Cohen et al. found erosive changes in 5% of hand or foot radiographs of 200 SLE patients with a minimum follow-up of at least 2 years (or until death). Erosive disease was seen especially in nonwhite women, and there was greater renal involvement and more widespread or severe disease in those with erosive arthropathy. The authors noted that anti RA-33 antibodies may identify those patients with SLE who are at risk for erosive disease. van Vugt et al. found that lupus patients who displayed erosion were positive for rheumatoid factor.
Erosive changes occur in a small percentage of patients with SLE. Magnetic resonance imaging (MRI) and ultrasound are generally more sensitive for finding erosion than is radiography.
MRI of Joints in SLE
MRI allows all portions of the joint and the periarticular soft tissues to be evaluated and is more sensitive for erosion than are radiographs. MRI evaluation of the hands of 14 patients with SLE by Ostendorf et al. showed periarticular capsular swelling in all cases, joint effusion in 7, and “edematous tenosynovitis” (fluid signal in tendon sheaths) in 6. Even in patients with deforming Jaccoud’s arthropathy of long duration or with higher seroactivity, there was no synovial membrane hypertrophy to indicate “active synovitis,” and only 1 patient showed mild synovial hypertophy.
MRI is apparently unable to differentiate patients with early SLE from those with early RA. Thus Boutry et al. compared the MRI findings in patients with inflammatory arthralgias later shown to have RA, SLE, or Sjogren’s syndrome. Radiographs showed no erosion. No significant difference was found among the groups in terms of global scores for synovitis, bone lesions, and tenosynovitis. The soft tissues adjacent to the joints of patients with SLE did not show abnormalities (such as has been seen in disorders such as psoriatic arthritis and reactive arthritis). Erosions were seen in SLE as well as in RA ( Figure 22-3 ).



Ultrasound of Joints in SLE
High-resolution ultrasound allows real-time assessment of joints and surrounding soft tissues at low cost and with no ionizing radiation. However, ultrasound examination requires an operator experienced in examinations of these musculoskeletal structures. Power Doppler is complementary, allowing hyperemia to be detected. Using these techniques, effusion, synovitis and tenosynovitis, tendon rupture, and bone erosion can be detected. Ultrasound is more sensitive for erosion than radiography and can be more sensitive than clinical examination. Evaluation of 17 patients with SLE and clinically involved hands found abnormalities on ultrasound, including tenosynovitis, tendon rupture, and erosions in the second or third MCP joints when no erosion was demonstrated on radiographs. Wrist evaluation in patients with SLE has shown a high incidence of synovitis and tenosynovitis using ultrasound.
Ultrasound can detect effusion, synovitis and tenosynovitis, tendon rupture, and bone erosion and is generally more sensitive than radiography or clinical examination for these findings.
Calcification
Periarticular calcification occasionally occurs in patients with SLE. Generally, when considering the causes of periarticular calcification, it is helpful to divide them into metabolic causes (abnormal calcium and/or phosphate levels) or dystrophic conditions (due to underlying tissue damage such as an underlying inflammatory process). A third category, idiopathic calcification, contains the specific entity of “tumoral calcinosis.” Among the causes of dystrophic calcification are progressive systemic sclerosis, mixed connective tissue disease, dermatomyositis and polymyositis, and SLE.
Calcium deposits in the skin (calcinosis cutis) may also occur in SLE ( Figure 22-4 ). Differential considerations for calcinosis cutis may also be grouped into metastatic, dystrophic, and idiopathic categories, as well as iatrogenic causes.


Causes of periarticular calcification can be grouped into (1) metabolic abnormalities (abnormal calcium and/or phosphate levels), (2) dystrophic calcification (due to underlying tissue damage such as an underlying inflammatory process), and (3) idiopathic calcification.
Cystic Changes
Well-defined radiolucent areas can be seen especially in the metacarpal heads and carpal bones of patients with SLE. Leskinen et al. found these lucencies in 41% of 125 patients with the disorder, and multiple lesions may be seen. The lesions are surrounded by a thin sclerotic rim of normal bone and may enlarge, increase in number and be associated with erosions. They are, however, nonspecific findings that may quite often be seen in unaffected individuals.
Tuft Resorption
Tuft resorption or periarticular calcification is seen in patients with SLE who have Raynaud’s phenomenon. Tuft resorption is a nonspecific finding and occurs also in conditions such as scleroderma, hyperparathyroidism, and psoriatic arthritis. These conditions can usually be differentiated on the basis of clinical and radiologic findings.
Acral Sclerosis
Osteosclerosis of the terminal tufts of the fingers is a nonspecific finding seen primarily in RA, scleroderma, dermatomyositis, and sarcoidosis as well as in normal individuals ( Figure 22-5 ). This abnormality was seen in 10 of the 59 (16.9%) SLE patients examined by Weissman et al. and was accompanied by Raynaud’s phenomenon in only 4 patients. Braunstein et al. found acral sclerosis in 12% of individuals with SLE presenting over the age of 50 years and in 10% of those presenting earlier in life.

Tendon Rupture
Tendon rupture is an uncommon sequela of SLE. It most likely is the result of inflammatory changes within the tendon but may also occur due to corticosteroid injections or trauma.
Osteonecrosis (Avascular Necrosis)
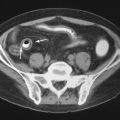
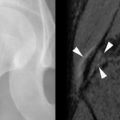
The association of SLE and avascular necrosis (AVN) was initially reported in 1960 by Dubois and Cozen. All but 1 of the 11 patients described had received corticosteroids, but in 5 patients it had been months or years since corticosteroid administration. The hips, knees, and shoulders are most commonly affected, although AVN of the small bones can also occur ( Figure 22-6 ). AVN can develop within the first months of initiation of high-dose corticosteroid treatment.



Only 5% to 10% of patients with AVN become symptomatic; therefore diagnosis cannot be based on the presence of symptoms. Jaovisidha et al. prospectively evaluated 11 patients with newly diagnosed SLE treated with corticosteroids. Despite being asymptomatic, MRI demonstrated AVN in 4 of 22 hips (2 of 11 patients). Early treatment may prevent collapse of the femoral head, and this suggests a role for screening studies such as MRI. Risk factors for asymptomatic AVN are said to be African-American ethnicity, Raynaud’s phenomenon, migraine headaches, and a maximum corticosteroid dose of at least 30 mg/day. Duration of treatment and cumulative dose seemed to relate to the development of clinically occult AVN in the study of Jaovisidha et al. The presence of bone marrow edema on MRI has been shown to correlate with the development of symptoms and the disappearance of edema with subsidence of symptoms. This finding has also been correlated with subsequent femoral head collapse ( Figure 22-7 ). In patients with symptomatic AVN, collapse of the femoral head usually occurs within 2 years.





MRI is useful in detecting early AVN, as changes on MRI may be visible before structural alterations (e.g., crescent sign, collapse) are evident. AVN may be present without symptoms.
Gastrointestinal Manifestations
Gastrointestinal (GI) manifestations of SLE are common and may affect any portion of the GI tract beginning with the mouth. Underlying causes of GI abnormalities include vasculitis, obliterative vascular thrombosis causing bowel ischemia, immune complex deposition producing an inflammatory response, and treatment complications. Abdominal pain occurs in up to 40% of patients with SLE and may be due to serositis, pancreatitis, bowel ischemia, or perforation.
Esophagus
Esophageal symptoms include dysphagia (incidence as high as 13%) and heartburn (incidence as high as 50%). Disordered esophageal motility is common, with decreased or absent peristalsis especially in the lower third of the esophagus. Possible causes of dysmotility include an inflammatory reaction in the esophageal muscles, ischemic vasculitic damage of the Auerbach plexus, or involvement of the vagus nerve. Symptoms may also be due to medications including nonsteroidal antiinflammatory medications or bisphosphonates. Esophageal ulceration or perforation may occur. Barium study can demonstrate abnormal motility and mucosal irregularity or ulceration associated with reflux ( Figure 22-8 ).

Stomach and Duodenum
NSAIDs and corticosteroids alone and particularly in combination may cause ulcer disease. In addition, high-dose corticosteroids may mask the early clinical signs of a perforated ulcer, necessitating vigilance on the part of the clinician and the imager. Double-contrast upper GI series can demonstrate ulcers.
Small and Large Bowel
Small-vessel vasculitis may lead to ischemic enteritis, bowel infarction with bleeding and/or perforation, and peritonitis. Clinical presentation is variable and nonspecific, and important and ominous physical findings may be masked by treatment with corticosteroids or immunosuppressant medications. Lee et al. noted enteritis (vasculitis) to be the most common cause of acute abdominal pain in patients with SLE.
Radiographs may show “thumbprinting” due to mural edema or hemorrhage but are less sensitive than computed tomography (CT) for the diagnosis of ischemia. Radiographs may also show free intraperitoneal air, pneumatosis cystoides intestinalis, ileus, or a pseudo-obstruction pattern. Gas in the portal venous system is an ominous sign.
Byun et al. note the importance of CT scanning for detecting the cause of GI symptoms and identifying complications such as perforation. These authors evaluated the CT findings in 33 patients (39 examinations) with SLE and acute abdominal pain. The diagnosis of ischemic bowel disease was made if at least three of the following signs were seen: bowel wall thickening, target sign, dilatation of intestinal segments, engorgement of mesenteric vessels, or increased attenuation of mesenteric fat. The areas of wall thickening could be multiple and did not necessarily correspond to a vascular territory (as would be true in large vessel disease).
The target sign in contrast-enhanced CT consists of thickened bowel wall with three layers: contrast-enhanced inner and outer layers and an interposed layer of decreased attenuation that is thought to be due to edema. It is a nonspecific finding in ischemia because it is seen in several “benign” conditions such as Crohn’s disease. It is not usually a feature of malignancy.
Ulcerative colitis, Crohn’s disease, collagenous colitis, celiac disease, and protein losing enteropathy may occur in SLE patients. Infectious colitis is a particularly important complication of SLE because it may be fatal, and this should be excluded before high-dose corticosteroid or immunosuppressive treatment is begun. Colon perforation may occur due to arteritis or diverticula, and the resulting pneumoperitoneum can be confirmed on upright or decubitus radiographs or on CT.
Gallbladder
Acalculous colicystitis may result from periarterial fibrosis and acute vasculitis. Ultrasonography or contrast-enhanced CT can show the resultant gallbladder wall thickening, as well as pericholecystic fluid.
Pancreatitis
Pancreatitis occurs in up to 28% of patients with SLE and is most often associated with active involvement in other organs. Findings on CT or ultrasound of acute pancreatitis are summarized by Lalani et al. as peripancreatic edema, phlegmon formation, infiltration of mesenteric fat, and sometimes enlargement of the affected portion of the gland. The pancreatic margins become indistinct. Chronic pancreatitis is common and may be asymptomatic. The gland saponifies and calcification forms within the ducts, which are irregularly narrowed and dilated on CT or ultrasound.
Renal Manifestations
Most patients with SLE have renal involvement. Lupus nephritis results from autoantibodies cross-reacting with glomerular surface antigens, mesangial matrix, or basement membranes, resulting in the deposition of immune complexes in the subepithelial and subendothelial layers of the glomeruli. Liberation of cytokines and glomerular necrosis and fibrosis ensue. As with other causes of medical renal disease, affected kidneys in SLE are hyperechoic on ultrasound examination and may be small.
Central Nervous System Manifestations
Central nervous system (CNS) abnormalities in SLE occur in approximately 40% of patients. They range from cognitive dysfunction and psychiatric disorders to seizures and strokes. Cognitive dysfunction is usually mild but is present in up to 30% of patients. Some instances of psychiatric symptoms may also be caused by high-dose corticosteroid treatment. Because lupus can involve any region of the nervous system such as the brain, meninges, spinal cord, and peripheral nerves, patients may present with stroke, demyelinating syndromes, headache, chorea, long tract signs, aseptic meningitis, myelopathy, Guillain-Barre syndrome, plexopathy, cranial and/or peripheral neuropathy, myasthenia gravis, autonomic disorder, or migraine. Lupus-related stroke occurs more often in young patients about 35 years old. Etiologies of stroke include coagulopathy associated with lupus anticoagulant, small vessel vasculitis, embolization from Libman-Sacks endocarditis, or even hypertension secondary to renal disease. In addition, accelerated atherosclerosis related to corticosteroid usage may be a contributing factor.
The diagnosis of SLE should be considered in any young patient who presents with stroke.
Infarcts in SLE are often small, involving deep or subcortical white matter, although occasionally they can be large and territorial ( Figure 22-9 ). Arterial or venous thrombosis, seen especially in patients with APS, can lead to serious morbidity and mortality.






Patients with neuropsychiatric disorders should be worked up to exclude underlying causes such as infection, medication effects, or metabolic disorders such as uremia or hypertensive encephalopathy. CT and MRI are useful imaging modalities to assist in the evaluation of these patients. Both unenhanced CT or MRI with GRE and diffusion sequences are particularly applicable for the assessment of hemorrhage and stroke ( Figures 22-10 to 22-12 ). A contrast study, in turn, will help detect infectious or inflammatory etiologies such as meningitis, abscess, or vasculitis.






Some of the CNS findings seen in SLE are shown in Table 22-3 . MRI has shown reduction of both gray and white matter volume in patients with SLE as compared with controls. Other MRI techniques such as magnetization transfer and diffusion weighted imaging (DWI) help to understand and define different aspects of the disease process. DWI in conjunction with apparent diffusion coefficient (ADC) maps describe the freedom (Brownian motion) of protons in water molecules to move within their environment (termed diffusivity ). Normally, diffusion is relatively impeded by physical barriers such as myelin sheaths. In cerebral ischemia, restricted diffusivity is thought to be caused by a relative decrease in extracellular water as it shifts to the intracellular space due to failure of sodium potassium pumps. This results in low ADC values with concurrent increased signal on DWI images (see Figures 22-11 and 22-12 ). In vasogenic edema, protons move freely along white matter fibers with increased ADC values and normal signal on DWI. There is also corresponding increased signal on T2-weighted and fluid attenuated inversion recovery (FLAIR) sequences due to the increased water content. DWI is especially important in differentiating lupus patients with ischemic changes (DWI positive) from those who have hypertensive encephalopathy (DWI negative) ( Figure 22-13 ). In addition, increased diffusivity has been found in patients with neuropsychiatric SLE thought to be related to demyelination or increased CSF space secondary to atrophy from the underlying disease process, corticosteroid usage, and, perhaps, neuronal/axonal damage.



Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree