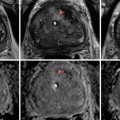
Fig. 8.1
Overdetection of insignificant prostate cancer
TRUS-guided biopsies miss clinically significant cancers. They have an estimated false-negative rate of 30–45 % [13, 14]. TRUS biopsy’s systematic error leads to significant cancer being missed as several parts of the prostate is systematically undersampled. First, the anterior part of the gland is missed as a result of its greater distance from the rectum (Fig. 8.2a). Second, areas in the midline are undersampled due to efforts to avoid the urethra. Third, the prostate apex is often inaccessible by the transrectal route [15–18].
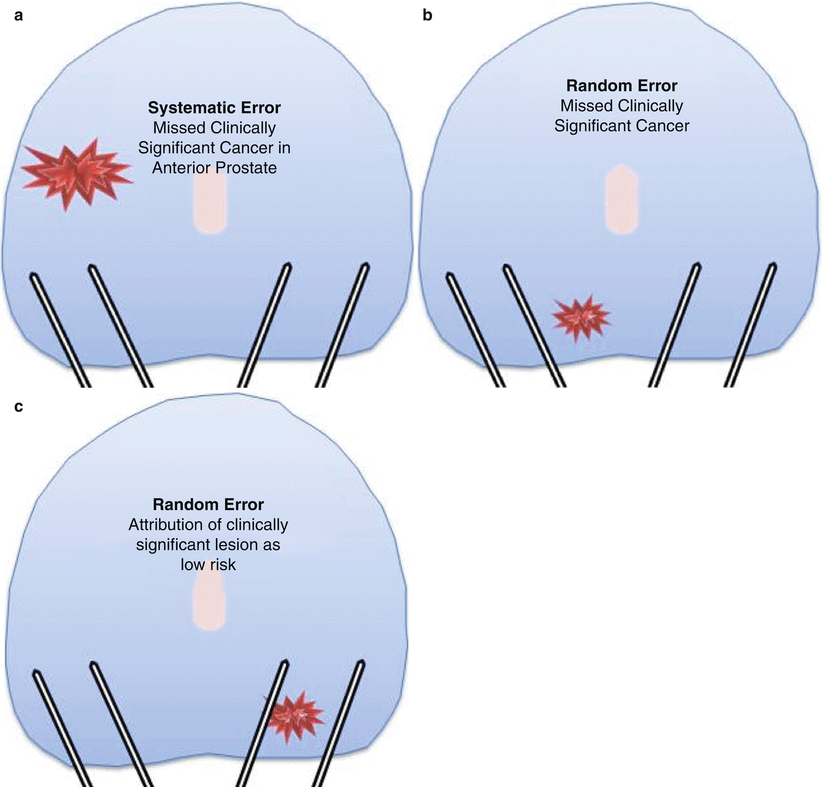

Fig. 8.2
TRUS biopsies underdiagnose clinically significant prostate cancer. (a) Random error. (b, c) Systematic error
TRUS-guided biopsies can be unrepresentative of the true burden of cancer. The random sampling error (Fig. 8.2 (b,c)) can mean that a biopsy does not hit the cancer lesion through its greatest diameter leading to either or both the size and the grade of cancer being underestimated [19] (Fig. 8.2c). Up to half of men deemed low risk on TRUS-guided biopsies can have higher burden or grade, or both, when a more accurate biopsy test is applied [16–18, 20–22]. As a result of the poor risk attribution, many men may not be able to receive the most suitable management in relation to their risk stratification.
TRUS-guided biopsy offers poor disease localization. As tissue preservative or focal therapy for prostate cancer depends on destroying the diseased tissue with minimal damage to healthy tissue, it is imperative to localize the diseased area in the prostate accurately to avoid undertreatment and recurrence on one hand and overtreatment and unnecessary increased morbidity on the other. TRUS biopsy is not able to provide such spatial information about the diseased area. And due to all above, the area diagnosed may not be the only area harboring clinically significant disease in the prostate.
Template transperineal prostate mapping (TPM) biopsies were conceived [23] to avoid the above issues and provide accurate risk assessment and location attribution of disease and have since gained increasing popularity and acceptance as a highly accurate diagnostic test [17, 18, 20, 21, 24].
Template Mapping Biopsies: Technique
Patient Preparation
In our routine practice at University College London, we do not prescribe preoperative preparation, although minimal bowel preparation with a phosphate enema the night before or on the day of the procedure significantly improves ultrasound imaging of the prostate. An alpha-blocker such as tamsulosin or alfuzosin, although not essential, may be prescribed prior to the procedure (2–7 days). It is strongly recommended to prescribe a 2-week course of tamsulosin postoperatively in order to minimize the risk of acute urinary retention. Seven days prior to the procedure, antiplatelet agents should be stopped. Anticoagulants should be stopped after consultation with local guidelines.
Patient and Equipment Setup
The patient is positioned in the lithotomy position and a short-term urethral catheter is usually fitted. The catheter’s main function is to delineate the urethra as a landmark to help align the prostate on ultrasound imaging and also to avoid injuring the urethra especially if it is deviated from the midline in nonsymmetrically enlarged glands usually due to benign prostatic hyperplasia. The catheter is spigotted, and the bladder should not be emptied to allow the bladder neck to be visualized on ultrasound. The scrotum and penis are taped to the abdomen. A biplanar ultrasound probe covered with an endocavity balloon mounted on a brachytherapy stepper is introduced into the rectum to visualize the prostate (Fig. 8.3). While inflating the balloon with water, it is essential to make sure most or all air bubbles are removed to avoid image artifacts (Fig. 8.4).
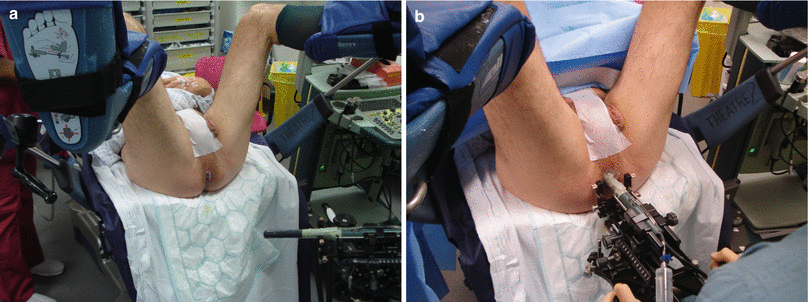


Fig. 8.3
Patient and probe setup. (a) Patient position. (b) Probe setup

Fig. 8.4
Probe in balloon cover with air bubbles emptied and sufficient gel coverage
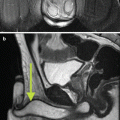
After the probe is introduced, the midline reference plane is established by visualizing the urethra and the catheter at the center of the sagittal plane or at the midline on the transverse plain on real-time ultrasound. This should be equivalent to the capital letter “D” on the 5 mm biopsy grid on transverse (axial) view (Figs. 8.5 and 8.6).
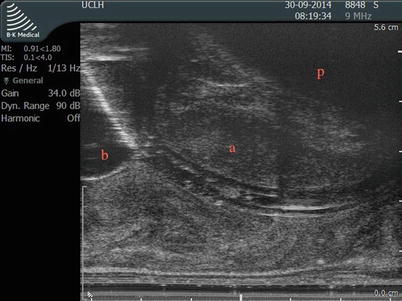

Fig. 8.5
Midline sagittal view of the prostate establishing the reference plain by visualizing the catheter in the urethra. kindly note the catheter balloon and the bladder base (b). The bladder base and the symphysis pubis (p) position should be considered while planning the biopsy. (a) represents the anterior area of the transition zone spared from biopsy to avoid urethral injury

Fig. 8.6
Transverse (axial) view of the prostate showing the catheter and urethra (arrow) aligned at the coordinates D3 in the middle of the grid which confirms the midline alignment, divides the prostate into anterior and posterior aspects, and allows the operator to plan the biopsy zones. Please note that the peripheral zone is well covered by the biopsy grid
It is important to ascertain that the posterior aspect of the prostate is included within the biopsy grid by moving the probe and stepper anteriorly or posteriorly and inflating/deflating the balloon (Fig. 8.6) to ensure adequate sampling of the peripheral zone specially at the level of the prostate base.
It is important to establish the position of the anterior aspect of the prostate in relation to the bony pubic arch to ascertain needle access to the anterior aspect of the prostate. Avoiding the pubic arch obstructing the needle path can be established by a combination of further extended hip flexion, balloon deflation, and stepper repositioning (Fig. 8.5).
Once an acceptable setup is achieved, the perineum is cleaned and the patient is draped. Shaving the perineum is optional. A sterile plastic drape and a brachytherapy type 5 mm sterile disposable grid are attached (Fig. 8.7) to the stepper. It is important that the template grid is calibrated to match the 5 mm grid seen on the ultrasound screen.
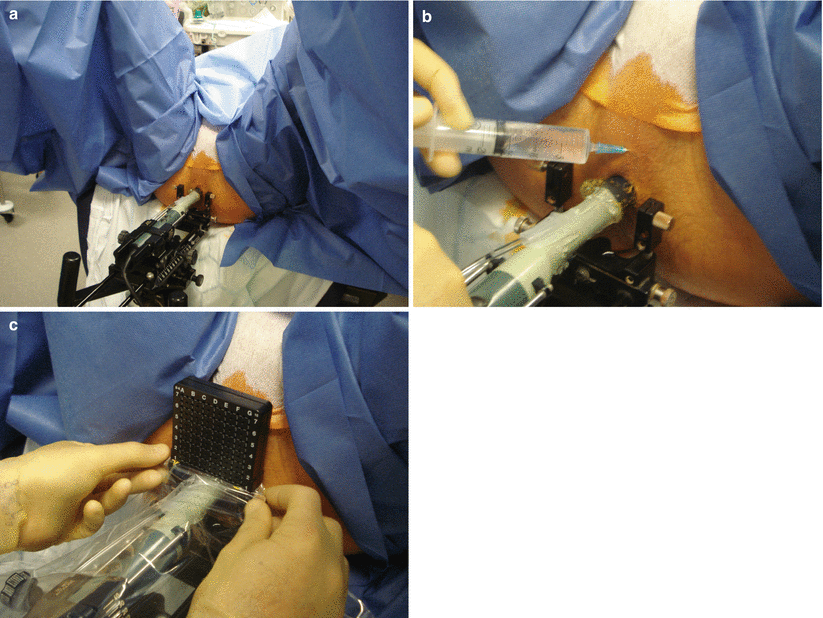

Fig. 8.7
Patient/probe preparation. (a) Patient draping and preparation. (b) Application of local anesthetic (bupivacaine with adrenaline 0.5 %). (c) Attachment of grid and plastic cover
Needle Deployment
On sagittal view, the prostate should be divided visually to cranial (basal) and caudal (apical) halves provided the prostate is large enough. The basal and the apical portions will be biopsied and potted separately. To perform a full template mapping biopsy, the biopsy needles are deployed through the template grid once apically and then basally before moving on to the next coordinate (hole) on the grid (Fig. 8.8) while avoiding injuring the urethra and the bladder. To avoid the urethra the midline plane anteriorly is always spared from biopsy (Fig. 8.5), which is usually the anterior coordinates along the D line in the grid. After obtaining a sample the needle should be rinsed in chlorhexidine and then in sterile water before it is reloaded and passed back to the operator. To save time two needles should be used in every procedure to allow the operator to biopsy while the sample is obtained from the other needle and allowing for it to be prepared, and reloaded for use. Figure 8.9 shows the scrub nurse’s equipment setup. Once the whole gland is sampled, the catheter is removed and the patient can be discharged on the same day provided they are voiding urine well with no large residual volumes.
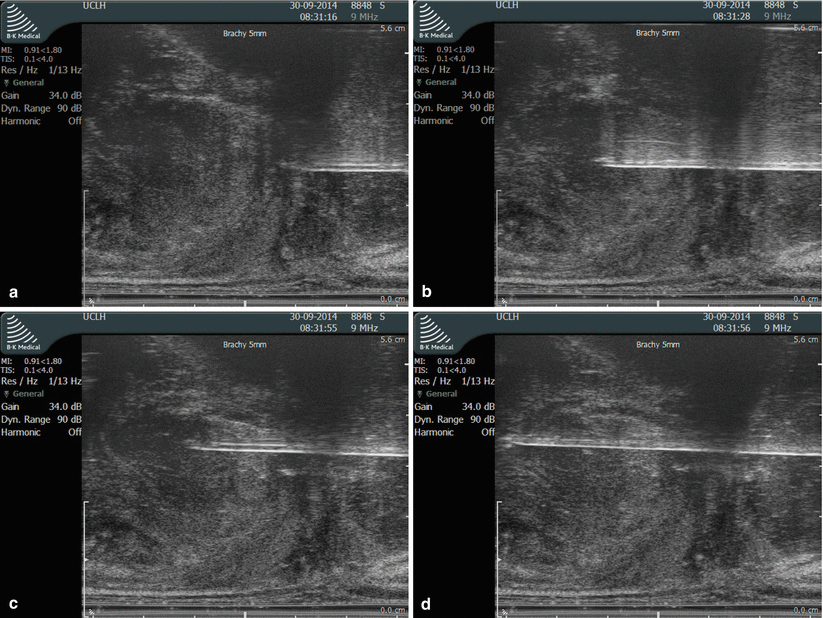

Fig. 8.8
Needle deployment. (a) Apical sampling: needle positioned against the apical capsule. (b) Apical sampling: needle deployed. (c) Basal sampling: needle positioned to sample the base where the previous biopsy tract ends, which can be tracked on the ultrasound screen and also felt as increased resistance once un-biopsied tissue is encountered. (d) Basal sampling: needle deployed stopping just short of the bladder base

Fig. 8.9
(a) Sterile water. (b) Chlorhexidine. (c) Plastic probe cover. (d) 5 mm brachytherapy grid. (e) Sterile gauze. (f) Drapes. (g) Superficial local anesthesia (bupivacaine with adrenaline). (h) Deep local anesthesia: used only in templates under local anesthetic or sedation (without adrenaline). (i) Biopsy 18 g needles
Assigning Zonal Anatomy to the Prostate
Biopsy cores can be labeled individually according to their X, Y coordinates on the grid and Z coordinates relating to whether it is basal or apical. The alternative is to divide the prostate into zones. There are also several ways to divide the prostate into anatomical zones. Barzell et al. initially divided the prostate into octants and then further divided them into three zones yielding 26 separate specimen pots [20]. Our group in University College London modified this further into the modified Barzell zones where the prostate is segmented into 20 zones (Fig. 8.10); then later reduced into 12 zones only to reduce the resource burden on pathology services (Fig. 8.11). The choice of approach should be driven first by the required accuracy of information and second by the amount of burden and logistics that can be tolerated. For example, if a TPM is used as a reference standard in a clinical trial validating a diagnostic modality like MRI as, for example, the PROMIS trial [25], a core-by-core coordinate record would yield the most accurate information at the expense of theater time, the need for logistical support, and histopathology processing cost. These issues can be mitigated using zonal sampling in which 5 mm sampling frequency is maintained but the cores geographically grouped and potted together. If the aim is for focal therapy, grouping the biopsies into zones should be sufficient to plan accurate treatment. In centers that only perform radical therapy, there is much less emphasis on the location of the disease and more on the risk stratification of the patient; hence, there will be no benefit in obtaining that level of detailed information.
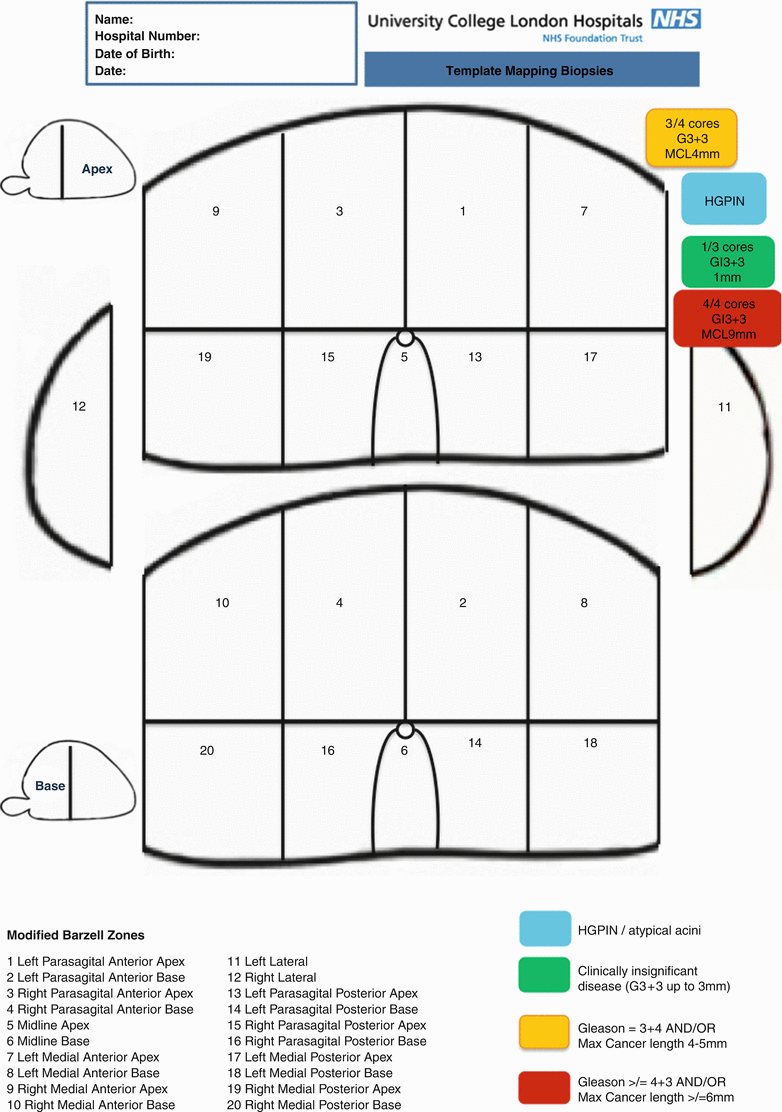
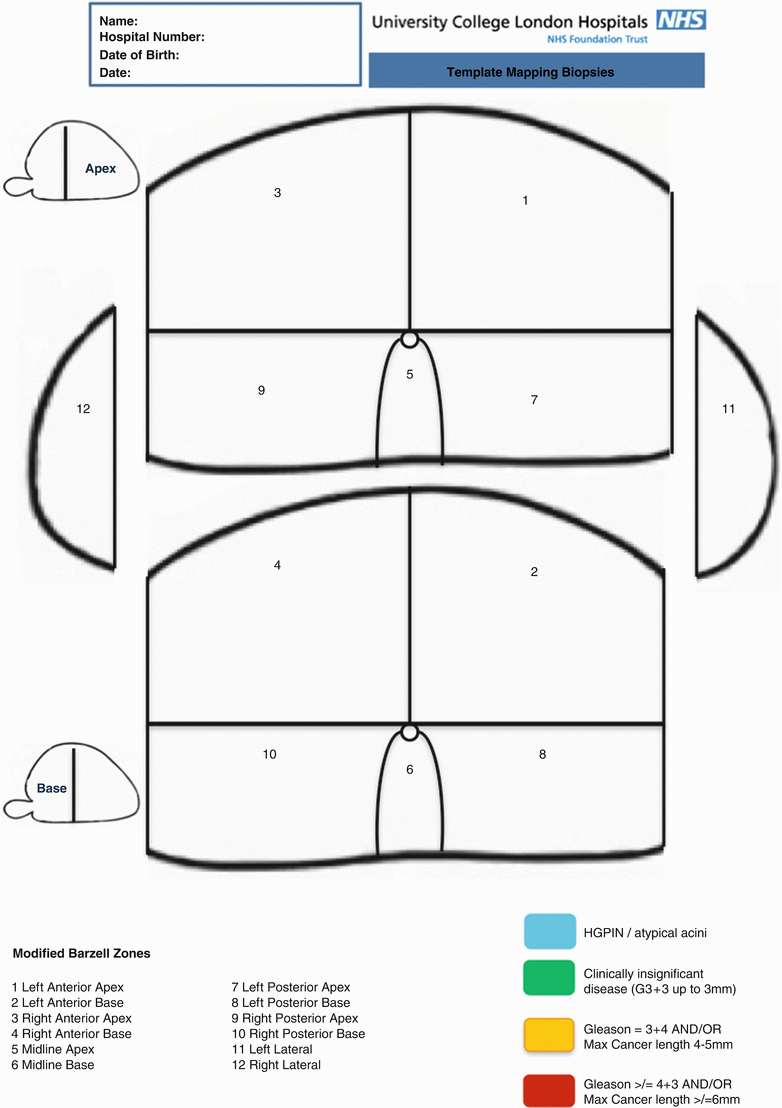

Fig. 8.10
Modified Barzell 20-zone approach similar to the approach used in PROMIS (Prostate MRI Imaging Study) and other diagnostic trials in UCL (Courtesy of University College London Hospitals, NHS Trust)

Fig. 8.11
Modified 12-zone approach applied in clinical practice in UCL (Courtesy of University College London Hospitals, NHS Trust)
Complications and Side Effects
With respect to morbidity and expected side effects, TPM biopsies have a similar side effect profile to that of TRUS-guided biopsy with three important differences. First, they carry a significantly lower risk of urosepsis (<0.5 %) – the most serious complication of TRUS-guided biopsy – as the needles do not traverse the rectal mucosa. Second, TPM biopsies confer a higher risk of self-limiting failure to void urine (5–10 %) as a result of greater gland swelling [26–28] compared to 1–2 % risk associated with TRUS-guided biopsy. Third, TPM biopsies require a general/spinal anesthetic, although in expert hands a limited mapping biopsy can be performed under sedation with local skin and periprostatic anesthesia [23]. On a more recent study by Losa et al. [29] comparing complication rates, according to the Clavien-Dindo classification, and changes in the quality of life, evaluated using the International Prostate Symptom Score, International Index of Erectile Function, and Functional Assessment of Cancer Therapy-Prostate questionnaires, before and 1 month after, template mapping biopsies did not appear to have a significant effect on the quality of life, and no life-threatening complications occurred, with negligible global Clavien-Dindo complication rates revealed.
The Diagnostic Performance of Template Mapping Biopsies
Template mapping biopsies demonstrate a high diagnostic yield (Table 8.1). This has been demonstrated by several publications. In a case series of 191 consecutive biopsy naive patients, Bittner et al. documented 73.3 % positive diagnoses with 24.3 % of these cancers in areas not typically sampled in a TRUS biopsy [30]. Symons et al. reported a series of 409 patients and showed an overall detection rate of 56.7 %. When stratified between those having a primary TPM and those having a repeat biopsy (after a previous negative biopsy), the detection rates were 64.4 % and 35.6 %, respectively, with significantly higher detection rates in prostates <50 mL in volume compared to larger prostates (65.2 vs. 38.3 %) [31].
Table 8.1
Variations in biopsy protocols and definitions of significance between publications
Study | Size | Age | PSA | Design | No. of cores | Technique | Definition | Measure | Results | Further info. |
|---|---|---|---|---|---|---|---|---|---|---|
Ong et al. 2015 [39] | 57 | 63a | 5.8a | Bx naive | 19a | Variable | GS≥7 or >3 cores positive | CDR | 53 % | 87 % of 53 % significant |
103 | 9.6a | negative TRUS | 24a | Variable | 36 % | 81 % significant | ||||
Katz et al. [38] | 17 | – | 5.6a | TPM/RP Validation | Density: no. of cores/volume a1.26 | Mapping | GS ≥ 7 or >50 %/core or >25 % Total/quadrant | Sensitivity and specificity | 86 % and 83 % | |
Nafie et al. 2014 [41] | 50 | 67b | 8b | Bx naive had TRUS then limited TPM | 36 cores per patient | Limited | Any cancer | CDR | 60 % positive, 32 % positive on both | No positive TRUS only. 28 % negative TRUS/+ve TPM |
Pepe et al. [42] | 226 | 64a | 7.6a | Mixed cohort. | Bx naive: 22 coresa Repeat biopsy: 32 coresa | Variable | Any cancer | CDR anterior zone | 11.8 % 8.8 % | |
Klatte et al. (2013) [43] | 50 | 57.5b | 7.3b | Prior negative TRUS biopsy | 24 cores per patient | Variable | Epstein criteria | CDR | 48 % | 25 % were insignificant |
Vyas et al. (2013) [44] | 634 | 63a | 7.66a | Mixed cohort | Volume: <30 mL = 24 cores >50 mL = 38 cores | Variable | Any cancer | CDR | Bx naive: 54 % Prior TRUS: 36 % |