6 Temporal Bone Trauma
The skull has been biomechanically adapted to resist extreme amounts of force relative to the appendicular skeleton,1 and the temporal bone is not an exception, with estimates of 1875 pounds of lateral impact required to generate a fracture within cadaveric temporal bone.2 Nonetheless, temporal bone injury, predominantly in the form of fractures, occurs with some frequency, estimated at 10 to 22% of closed head injuries.3,4 In a recent review of 820 fractures,5 the most common cause of temporal bone fractures was automobile accidents, followed by assault, falls, and motorcycle accidents. Men were involved in 76% of cases. In the pediatric population, motor vehicle accidents and falls were the most common causes of temporal bone fractures.6 Complications of temporal bone injury include hearing loss, facial nerve palsy, and vertigo. Additional complications are related to the adjacent intracranial compartment and include cerebrospinal fluid (CSF) leak, meningitis, and contusion.7 This chapter is organized into a discussion of normal anatomy and pseudofractures, temporal bone fractures, and complications of temporal bone injury.
Normal Anatomy and Temporal Bone Pseudofractures
The complex temporal bone anatomy and its relationship with adjacent bones of the skull create several normal linear structures on cross-sectional imaging that can mimic temporal bone fractures, referred to as pseudofractures.8 The interpreting radiologist should have a good working knowledge of the normal appearance of these structures, which have previously been classified into extrinsic fissures and sutures, intrinsic fissures, and intrinsic channels.
Extrinsic fissures and sutures separate the temporal bone from adjacent bones and can often be differentiated from fractures by their irregular course and the presence of sclerotic, corticated margins; these structures include the temporoparietal suture, the petrooccipital fissure, the sphenopetrosal fissure, and the occipitomastoid suture. The temporoparietal suture is best visualized on axial computed tomography (CT) images as a cleft in anteroposterior (AP) orientation along the squamosal portions of the temporal bone, with some overlap of the superior margin of the squamosal portion of bone (Fig. 6.1A). The petrooccipital fissure is best noted on axial imaging (Fig. 6.1B), originating from the petrous apex anteriorly and medially and bounded posterolaterally by the jugular foramen. It contains cartilaginous material as well as the inferior petrosal sinus, along its course from the cavernous sinus to the pars nervosa of the jugular foramen. More anteriorly are the shorter sphenopetrosal fissures, seen along the floor of the middle cranial fossa between the posterior edge of the greater wing of the sphenoid bone and the petrous apex; these are also called the angular or petrosphenoidal fissures and are best visualized in patients with a nonpneumatized sphenoid (Fig. 6.1C). The occipitomastoid sutures (Fig. 6.1B and Fig. 6.1G) can be seen at the posterior margin of the mastoid process, and can be quite asymmetric and irregular in appearance. Additional clefts can be seen at this region due to mastoid emissary veins.
Recall that the temporal bone is made up of five portions (see Chapter 3). Intrinsic fissures, formed at sites of apposition of these various portions of the temporal bone, include the petrosquamosal fissure, petrotympanic fissure, tympanosquamous fissure, and tympanomastoid fissure. The petrosquamosal fissure varies in prominence but can sometimes be seen as a tiny defect within the tegmen tympani on coronal images, or as a small oblique cleft extending anteromedially from the glenoid fossa toward the greater wing of sphenoid, seen on axial images of the skull base (Fig. 6.1C). Körner’s septum represents a continuation of this fissure (Fig. 6.1D); this is seen along lateral margins of the mastoid antrum, with a thicker continuation of this septum variably seen extending inferiorly into the mastoid process (Fig. 6.1E). The petrotympanic (glaserian) fissure is poorly visualized on routine axial and coronal images due to its obliquity, but it can be seen on sagittal images (Fig. 3.9B), its contents including the chorda tympani nerve and the anterior tympanic artery, a branch of the internal maxillary artery supplying the middle ear. The tympanosquamosal and tympanomastoid fissures are best seen within the anterosuperior and posterosuperior walls of the external auditory canal, respectively, potentially mimicking EAC fracture (Fig. 6.1B, Fig. 6.1F, and Fig. 6.1G). Medially, the tympanosquamosal fissure divides into the petrotympanic and petrosquamosal fissure.
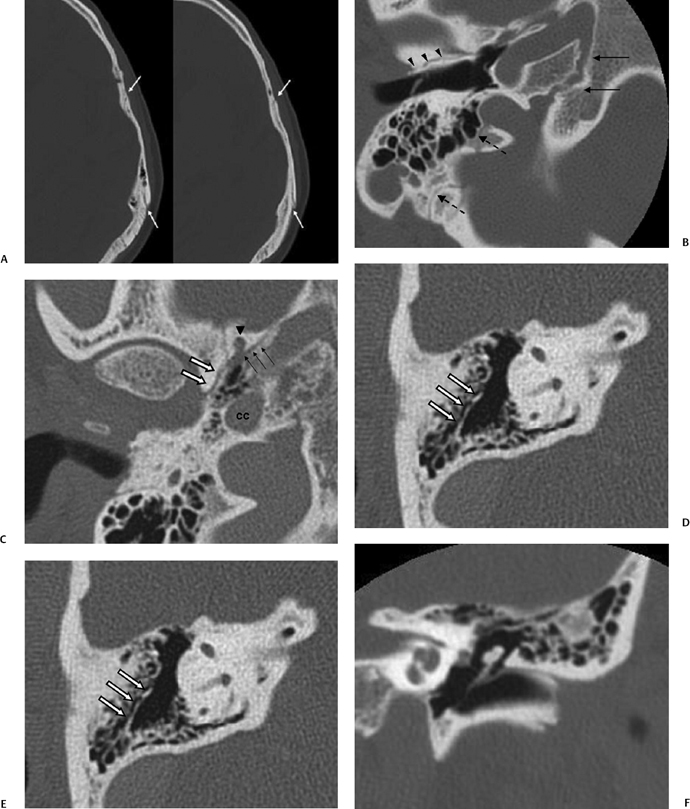
Fig. 6.1 Pseudofractures. (A) Axial computed tomography (CT) image. The temporoparietal suture is seen paralleling the outer table of the calvarium (arrows). (B) Axial CT image. The petrooccipital fissure (long arrows), occipitomastoid suture (dashed arrows), and tympanosquamous fissure (arrowheads) are depicted. (C) Axial CT image. At the basal skull, anterior portions of the petrosquamosal fissure (white arrows) can be seen extending anteromedially from the glenoid fossa. The sphenopetrosal fissure (thin black arrows) is seen between petrous bone and the posterior margin of the greater wing of the sphenoid. Also noted on this image are the foramen spinosum (arrowhead) and the carotid canal (cc). (D) Axial CT image. Körner’s septum (arrows) is seen along the lateral margins of the mastoid antrum, representing a plane of fusion between the petrous and squamous portions of the temporal bone. (E) Axial CT image. A continuation of this septum (white arrows) along the plane of the petrosquamosal fissure is seen extending inferiorly into the mastoid process, thicker than that seen in Fig. 6.1D. (F) Coronal CT image. The tympanosquamous fissure is seen paralleling the external auditory canal at its superior aspect.
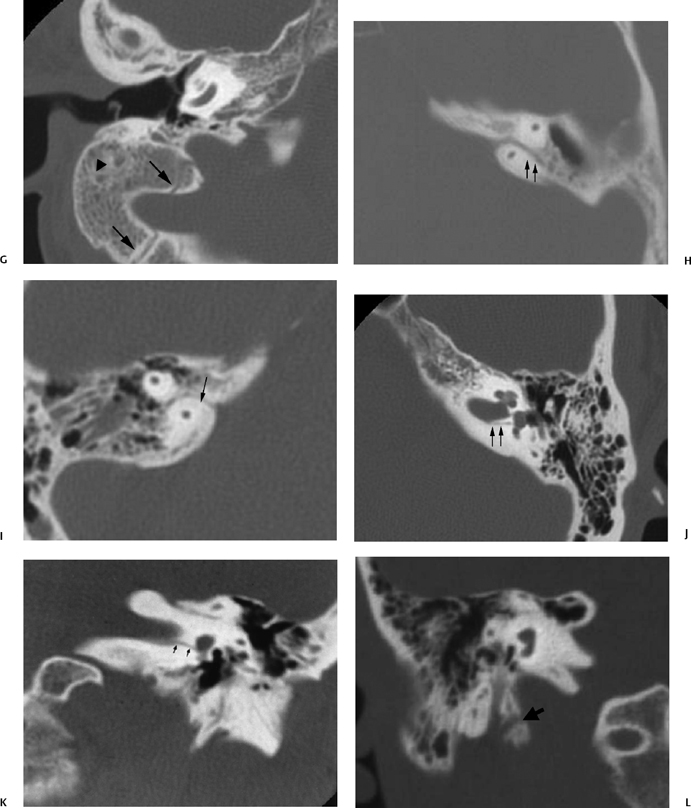
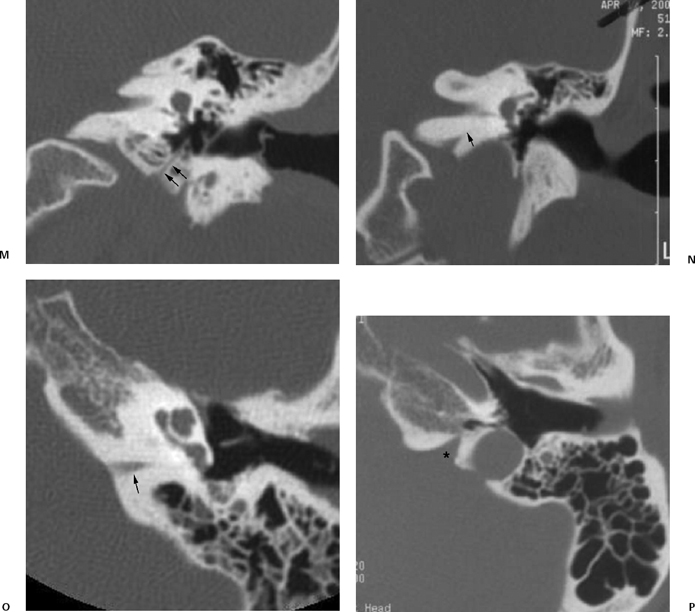
Fig. 6.1 (Continued) (G) Axial CT image demonstrates the tympanomastoid fissure (arrowhead) and the occipitomastoid fissure (arrows). (H,I) Petromastoid canal. Axial CT images demonstrate curvilinear course of the petromastoid canal (arrows) containing the subarcuate artery (J,K) Singular canal. (J) Axial and (K) coronal CT images. Narrow channel posteroinferior (arrows) to the internal auditory canal transmitting the posterior ampullary nerve to the posterior semicircular canal. (L) Mastoid canaliculus. Coronal CT images (different patients) demonstrate a channel extending inferolaterally from the jugular foramen to the mastoid segment of the facial nerve canal. This canaliculus (arrow) contains the nerve of Arnold (branch of vagus nerve).
Intrinsic channels, which can mimic temporal bone fractures, include the petromastoid canal, singular canal, mastoid canaliculus, and inferior tympanic canaliculus, as well as vestibular and cochlear aqueducts, the latter described in Chapter 5 (Fig. 6.1N and Fig. 6.1O).
The petromastoid canal (PMC), averaging 1 mm in diameter and 6 mm in length (Fig. 6.1H and Fig. 6.1I), is a remnant of the subarcuate fossa, a large structure within the fetus that extends from the vestigial mastoid to the posterior fossa.9 Within the first 5 years of life, it narrows to form its distinctive anteriorly convex curvilinear course on axial images just beneath the superior semicircular canal (SSCC).10 It is a useful surgical landmark, lined by dura and containing the subarcuate artery and vein. The PMC is a potential source of dural tear, CSF leak, and subsequent meningitis, as it is a site of communication between the mastoid antrum and the intracranial cavity.1,11,12
The singular canal is visualized on axial and coronal CT images (Fig. 6.1J and Fig. 6.1K). It transmits the posterior ampullary nerve to the posterior semicircular canal (PSCC), taking a posterolateral course from the internal auditory canal (IAC). It is a fairly narrow structure, 0.5 mm in diameter and 2.5 to 4.0 mm in length.13
The inferior tympanic canaliculus (ITC) (Fig. 6.1M) is best identified on coronal images obliquely coursing between the hypotympanum and jugular foramen containing the inferior tympanic branch of the glossopharyngeal nerve (nerve of Jacobson, CN IX). The aberrant internal carotid artery enters the tympanic cavity via the ITC (see Chapter 4).
The mastoid canaliculus (Fig. 6.1L) courses mediolaterally between the jugular foramen and the descending facial nerve canal, and as such can be seen on axial or coronal CT imaging. Transmitted through this channel is the nerve of Arnold, a vagus nerve branch providing sensation to a portion of the external auditory canal and middle ear.
The vestibular and cochlear aqueducts are considered in detail in Chapter 5 and will not be discussed in detail here. They could be confused with fractures by the novice observer.
The glossopharyngeal sulcus is consistently seen inferior to the cochlear aqueduct on axial CT images (Fig. 6.1P) and contains the glossopharyngeal nerve as it passes from the medulla to the pars nervosa of the jugular foramen.
Temporal Bone Fractures
Traditional Classification
The traditional classification of temporal bone fractures was organized with reference to the orientation of the fracture line relative to the long axis of the petrous bone, with fractures divided into longitudinal and transverse types.14–19 Given this orientation, axial images are helpful in delineating the course of these fractures because of their excellent depiction of the long axis of petrous bone. The intrinsic strength of the temporal bone is such that fractures traversing it will commonly extend between sites of least resistance, typically intrinsic foramina and cavities.
Recently, with the adoption of high-resolution computed tomography (HRCT) as a routine imaging tool in temporal bone trauma, it has become apparent that many fractures have features of both longitudinal and transverse types and may be classified as complex or mixed (Fig. 6.2).20–24
Longitudinal Fractures
Longitudinal fractures are those oriented parallel to the long axis of petrous bone. These are noted in multiple series to represent the majority of temporal bone fractures, constituting 70 to 90%. Fractures typically result from a lateral blow to the temporoparietal region and are commonly associated with fractures of the squamosal portion of the temporal bone (Fig. 6.3, Fig. 6.4, Fig. 6.5, and Fig. 6.6).18,20,25 Subtypes of the longitudinal fracture include anterior and posterior varieties, in reference to the lateral origin of the fracture line.26 Posterior longitudinal fractures arise in the mastoid process or posterior squamosa, extending anteromedially toward the foramen lacerum, and commonly involving the ossicular chain and first genu of the facial nerve. Anterior (oblique) longitudinal fractures arise from the anterior squamosa and extend medially toward the petrous apex, spanning the tegmen tympani and facial nerve canal anterior genu, often involving the glenoid fossa of the temporomandibular joint (Fig. 6.7).26,27 This subtype of fracture can be complicated by epidural hematoma resulting from middle meningeal artery disruption.
Longitudinal fractures typically spare the otic capsule, with the natural path of least resistance extending anteromedially to the petrous apex.28 Fracture lines can extend to the carotid canal with subsequent vascular injury (Fig. 6.8; see subsection Brain and Vascular Injury below). Longitudinal fractures can also extend to involve the contralateral temporal bone across the sphenoid.17,29
Sudden momentary displacement of the petrous apex can result from disruption of the sphenopetrosal junction (petrooccipital synchondrosis),28 an occurrence more common in children due to increased flexibility of the osseous structures and the unfused status of the synchondrosis. When this displacement is anterior, carotid artery tears and traumatic arteriovenous fistulas are potential complications; brainstem and cerebellar contusions are more common in the setting of posterior displacement.28 Magnetic resonance venography (MRV) is recommended in cases of associated sigmoid sinus plate violation, as this can herald direct venous injury.
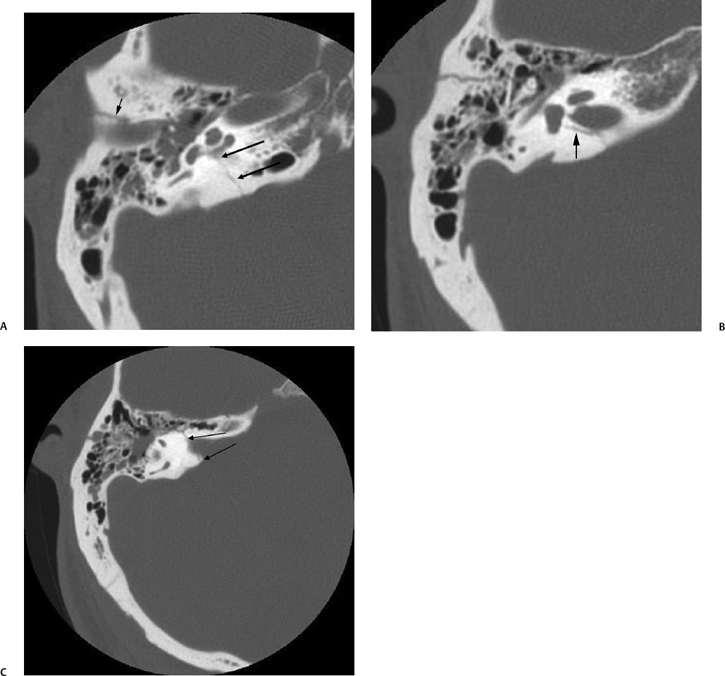
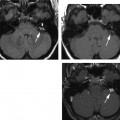
Fig. 6.2 Mixed temporal bone fracture. (A) Axial computed tomography (CT) image demonstrates transverse fracture line extending to involve the basal turn of the cochlea (double arrows). Hemotympanum is present. Additional longitudinal fracture component is noted (short arrow). (B) Axial CT image, more superior. The longitudinal fracture is better visualized. Note the singular canal, which mimics a fracture in this case (long arrow). (C) Axial CT image, more superior. Transverse fracture line (long arrows) crosses the internal auditory canal.
A rare variant of the longitudinal fracture is known as the floating cochlea, separation of the petrous apex from its lateral and inferior attachments.30 In this setting, the middle ear is a plane of the cleavage, separating the petrous apex from mastoid portions of the temporal bone. As the fracture lines run lateral to the otic capsule, the labyrinth is preserved, and complete sensorineural hearing loss (SNHL) does not typically occur. This injury is, however, associated with immediate conductive hearing loss (CHL), due to ossicular disruption, as well as abducens and facial nerve palsies. The sixth nerve palsy is due to rotation of the petrous apex with resultant compression of Dorello’s canal. Again, this type of fracture is most likely to result in the pediatric population due to increased flexibility of the skull.
Longitudinal fractures extending through the tympanic annulus are usually associated with tympanic membrane injury and hemotympanum, resulting in CHL.15,25 Ossicular injury is suspected if CHL does not improve following healing of the tympanic membrane and resolution of hemotympanum (see subsection Hearing Loss below).
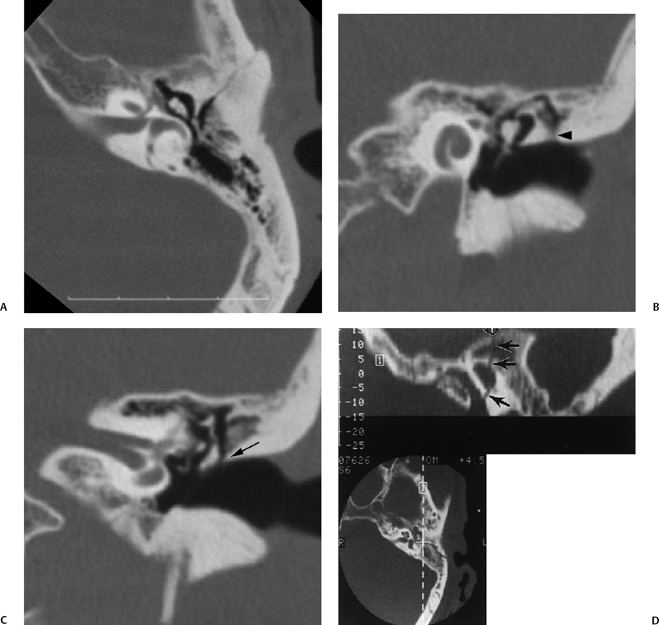
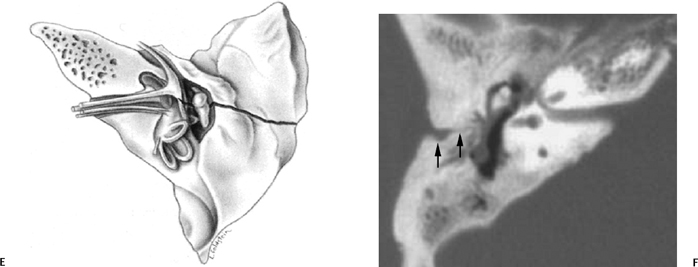
Fig. 6.3 Longitudinal fracture and cerebrospinal fluid (CSF) otorrhea. (A) Axial computed tomography (CT) section. Anterior subtype fracture line is actually oblique rather than truly longitudinal. (B,C) Corresponding coronal CT images. Fracture through the scutum (arrow). (D) Different patient, sagittal reconstruction. Longitudinal fracture line (arrows). Ossicles appear spared, and there is complete opacification of the entire middle ear and mastoid and profuse CSF otorrhea. Note the fracture line extending to the tegmen (arrowhead). (E) Artist’s rendering of conventional longitudinal fracture line. (F) Additional patient, axial CT image, posterior subtype longitudinal fracture line (arrows).
Individuals with longitudinal fractures are also at increased risk of developing acquired cholesteatoma in the delayed setting (Fig. 6.9), resulting from ingress of squamous epithelial debris into the middle ear through the fracture defect, as endochondral bone is limited in its ability to form callus.16,31–34 These cholesteatomas can be aggressive and difficult to manage surgically.
CT has become essential in diagnosing longitudinal fractures.20–22,35 Magentic resonance imaging (MRI) may demonstrate hemorrhagic products or fluid,7 but less commonly identifies longitudinal fracture lines, with sensitivity 66% in one series.36 Dural enhancement at the rostral aspect of the injured temporal bone was identified in 68% of patients in the same series, regardless of whether or not a fracture line was visible; although histologic correlation was not described, it was postulated that this may be due to dural microtear or temporal bone microfracture.36 SNHL resulting from longitudinal fracture is most often related to labyrinthine concussion as direct labyrinthine involvement by longitudinal fractures is rare.14,15,18
Transverse Fractures
Transverse fractures are significantly less common than their longitudinal counterpart. Historically, reported frequency is 20%, but more recent studies suggest a range from 2.5 to 5.6%.5,37 This may reflect both a decrease in the severity in the injuries currently reported and an increase in the detection of less severe fractures, which tend to be longitudinal in nature. Transverse fractures run perpendicular to the long axis of petrous bone, typically resulting from frontal or occipital impact. These fractures extend from the jugular foramen and foramen magnum to the middle cranial fossa, commonly passing through the vestibular aqueduct (Fig. 6.6, Fig. 6.10, Fig. 6.11, and Fig. 6.12).15,16,27,38–40
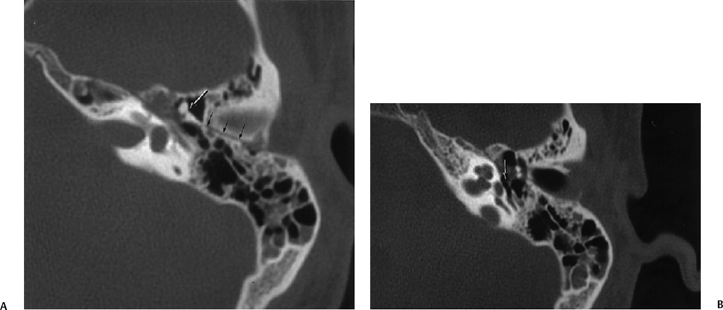
Fig. 6.4 Longitudinal fracture, malleoincudal disruption, facial weakness. (A) Axial computed tomography (CT) image. Longitudinal fracture (small black arrows). There is a partial subluxation of the malleoincudal articulation (arrow) with counterclockwise rotation of the incus body. The short process is rotated toward the fracture line rather than its normal orientation toward the fossa incudis. Medial to the malleus head there is some abnormal soft tissue presumably representing hematoma in direct apposition to the first genu of the facial nerve canal. Some debris is inside mastoid air cells. (B) Axial CT, more inferior. Intact stapes super-structure (arrow). This is important in operative planning.
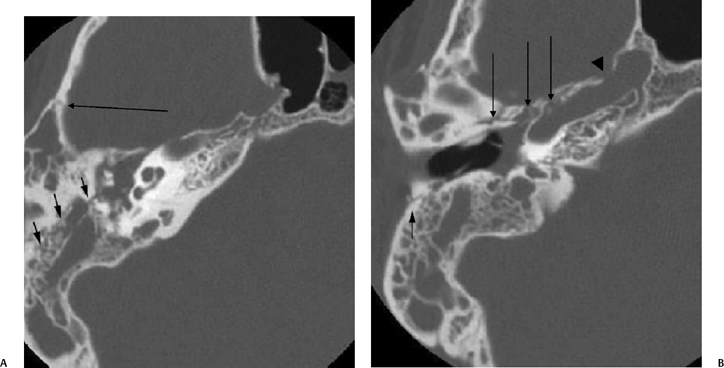
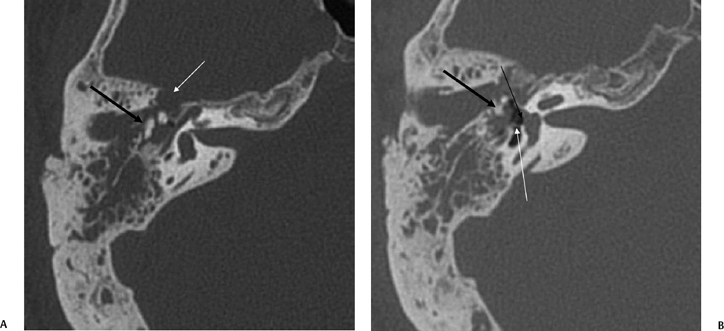
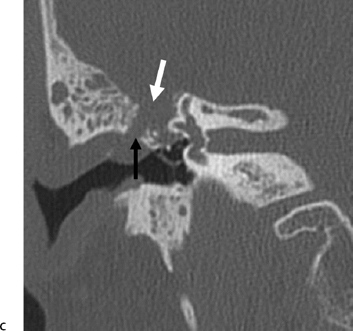
Fig. 6.6 Complex fracture, ossicular disruption, tegmen defect. (A) Axial computed tomography (CT) image, complete incus dislocation (black arrow). Tegmen defect (white arrow). (B) Axial image (more inferior). Complete incus dislocation (black arrow). Oval window disruption (thin black arrow). Stapes malrotation (white arrow). (C) Coronal CT image. Tegmen defect (white arrow). Fractured scutum (black arrow). Due to tegmen defect, magnetic resonance imaging was strongly recommended to evaluate for encephalocele. Expansile nature of some of the middle ear debris is reminiscent of posttraumatic cholesteatoma. Patient refused surgery and further evaluation.
Medial and lateral subtypes have been described, with lateral transverse fractures involving the otic capsule and often resulting in complete sensorineural hearing loss. Lateral transverse fractures can also be associated with stapes footplate injury, which may result in SNHL secondary to perilymphatic fistula formation. Medial transverse fractures typically cross the IAC at the fundus. In this group of fractures, SNHL is variable, but can be complete with direct cochlear nerve injury or transection.26,41 A
case of fatal exsanguination from medial transverse fracture involving the carotid canal has been reported.42
Classifications
In 1992, Ghorayeb et al43 reviewed cases of HRCT with three-dimensional reconstruction and described in detail the oblique fracture classification. Typical longitudinal fractures travel vertically within the petrotympanic fissure; oblique fractures were seen to cross the petrotympanic fissure in a more horizontal plane, extending superiorly to involve the middle cranial fossa. The clinical features of these oblique fractures are similar to those seen in longitudinal fractures.
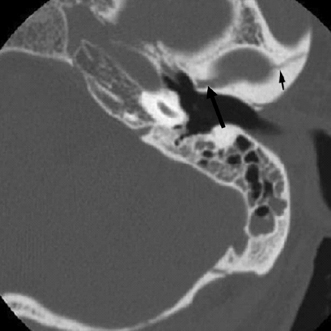
Fig. 6.7 Oblique fracture. Fracture line (arrows) is seen to extend through the glenoid fossa of the temporomandibular joint. Mastoid air cell opacification is noted.
Some authors have argued for the adoption of an alternate classification scheme into fractures involving the otic capsule and those sparing the otic capsule.44 Multiple studies have confirmed that fractures involving the otic capsule result in greater risk for CSF fistula formation and facial nerve injury, as well as the development of SNHL. In the series of 820 patients reported by Brodie et al, only 2.5% of fractures violated the otic capsule.5 These patients also have an increased incidence of intracranial complications, including intracranial hemorrhage.37
Yanagihara et al45 analyzed 97 fractures and have devised another system of classification in an attempt to predict specific portions of the facial nerve at risk and surgical intervention required. Type 1 fractures travel across the mastoid process, mainly involving the mastoid segment of the facial nerve. Type 2 fractures extend across the mastoid process to the external auditory canal, typically involving the nerve at the tympanic or mastoid segment. In type 3 fractures, the fracture line extends across the mastoid cortex and external auditory canal to the tympanic portion of the facial nerve. These are often associated with ossicular injury. Type 4 fractures travel across the tegmen tympani and antrum, involving the geniculate ganglion. This is subdivided into type 4A (without involvement of the inner ear and IAC) and 4B fractures. The latter fractures involve the labyrinthine and tympanic segments and can be complicated by loss of lacrimation due to injury to the greater superficial petrosal nerve. These patients are also prone to CSF otorrhea.
It should be noted that these classification systems are not mutually exclusive, each addressing separate key features of temporal bone fractures that need to be considered in radiologic interpretations of temporal bone imaging studies, including morphology (longitudinal versus transverse), labyrinthine involvement (otic capsule sparing versus otic capsule violating), and site of potential facial nerve injury (Yanahigara classification).
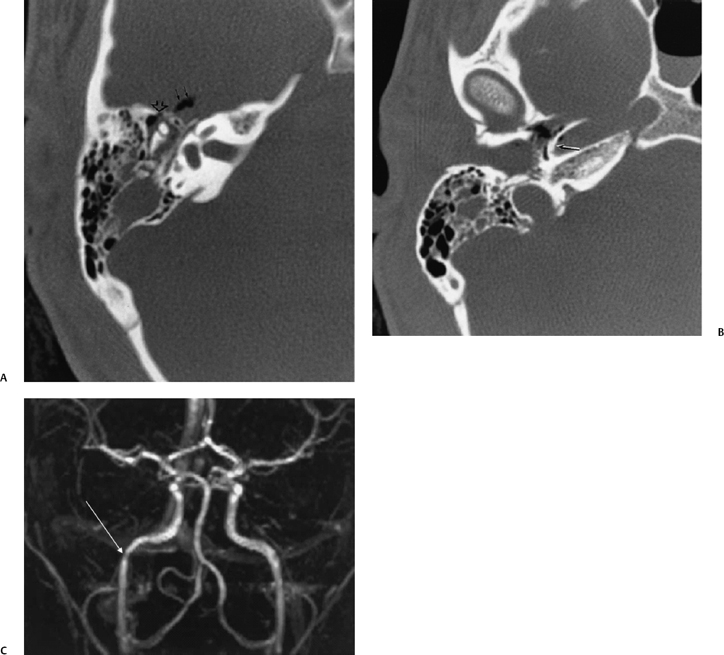
Fig. 6.8 Fracture of the anterior portion of the tympanic cavity and carotid canal. (A) Axial computed tomography (CT) image at level of malleoincudal articulation. Disruption of the anterior wall of the epitympanum (open arrowhead). There is a pneumocephalus (double solid arrows). There is patchy debris presumably present and a hematotympanum through the attic and antrum. (B) Axial CT image, more inferior. There is bony disruption of the posterolateral wall of the horizontal segment of the canal (arrow). Note the tiny focus of air within the carotid canal, which is very abnormal. There is also a fracture of the external auditory canal with debris through this structure. (C) Three-dimensional phase contrast magnetic resonance angiography sequence. Pathological narrowing of the horizontal portion of the carotid canal (arrow) is evident. This could represent a dissection, but more likely it represents extrinsic narrowing secondary to the presence of extramural edema and hemorrhage.
An additional fracture involving the temporal bone is the fracture of the styloid process (including fracture of ossified stylohyoid ligament).46,47 This typically presents with upper cervical pain, although temporal headache and temporomandibular joint complaints are also reported. Fractures of the tympanic ring and temporomandibular joint can result from direct impact to the mandible described in chin-dashboard trauma, typically in the setting of motor vehicle accidents. This results from impaction of the mandibular condyle against the anterior tympanic spine, the inferior boundary of the petrotympanic fissure.
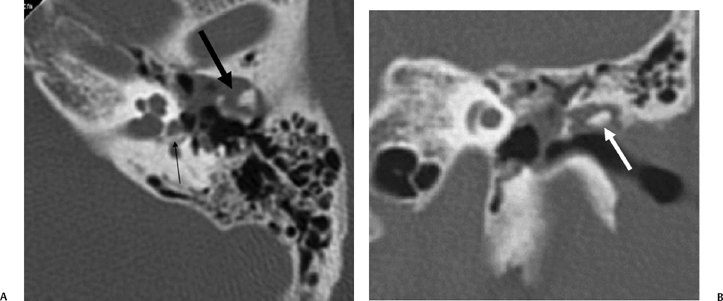
Fig. 6.9 Complex fracture, ossicular dislocation, Oval window disruption, posttraumatic cholesteatoma. (A) Axial CT image through the epitympanum. Ossicular chain is “exploded” (thick arrow). There is a nondependent expansile soft tissue mass. A bony fragment (ossicle) has penetrated the oval window and resides in the vestibule (arrow). (B) Coronal CT image. Dislocated incus (arrow) has penetrated the tympanic membrane and resides in the external auditory canal.
Pathologic Conditions Associated with Temporal Bone Trauma
Facial Nerve Injury
Posttraumatic facial nerve injury is the second most prevalent cause of facial nerve dysfunction following Bell’s palsy.15,16,48–54 In the setting of paresis or paralysis, lesions of the facial nerve can be localized by evaluation of adjunctive symptoms related to its branches, such as taste, stapedius function, and lacrimation (see Chapter 7). Injury to the facial nerve can result from intraneuronal hematoma, complete transection (neurotmesis), exposure and compression (neuropraxia), and stretching and crushing injuries (axontomesis) (Fig. 6.6, Fig. 6.13, Fig. 6.14).55 Transection of the nerve is associated with immediate and complete facial nerve paralysis. Facial nerve palsy was noted to occur in ∼20% of longitudinal fractures and ∼50% of transverse fractures.48,56 More recent data demonstrate a lower rate of posttraumatic facial nerve palsy, resulting from increased utilization of seatbelts and airbag technologies, decreasing the severity of motor vehicle trauma.57 Increased detection of less severe fractures by HRCT, which would have normally remained clinically occult, may also contribute to this apparent decrease. In Brodie’s series, 7% of fractures resulted in facial nerve palsy, with approximately one quarter of these being immediate in onset.5 In a pediatric series,6 the incidence of facial nerve paralysis was only 3%; this difference may relate to decreased ossification and increased flexibility in the pediatric skull.58,59
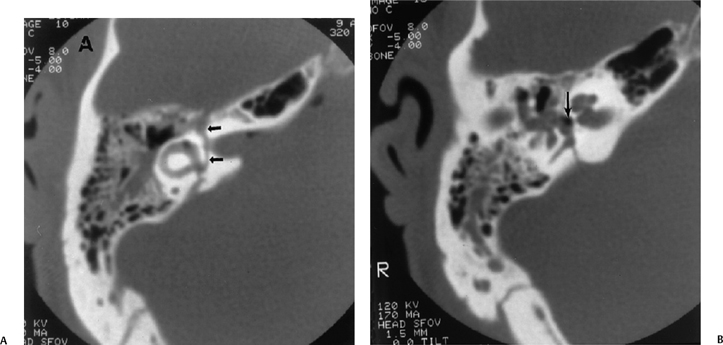
Fig. 6.10
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree