The Altered Breast
EXCISIONAL BIOPSY CHANGES
Accurate preoperative wire localization methods now facilitate minimal volume biopsies, and an increasing number of minimally invasive imaging-guided needle biopsies have reduced the need for excisional biopsies. Consequently, the likelihood of an excisional biopsy creating a permanent change that affects subsequent mammographic interpretations has decreased significantly. When postoperative changes occur, they often resolve in the first several months after the procedure. Unless a mammogram is done in the first 6 months after the biopsy, annual mammograms are normal in more than half of these women (1, 2, 3).
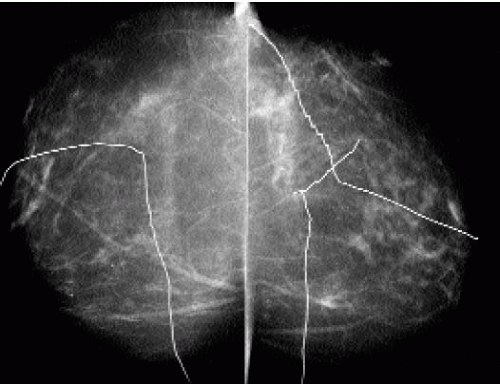
The technologists take a careful history, inspect the breast, and document on the patient’s history sheet the site of any scars . This allows the interpreting radiologist to correlate any findings on the mammogram with prior biopsy sites. Although we do not routinely mark biopsy scars at the time screening studies are done, thin wires or metallic BBs can be used (2). Given the expense associated with these markers, in conjunction with the high number of women who have had at least one breast biopsy, we reserve the use of markers for the few patients a year in whom there is a question about an area of mammographic concern correlating with a biopsy site. Also, having one or multiple linear markers on a study is a source of distraction that is not necessary for most patients (Figure 10.1).
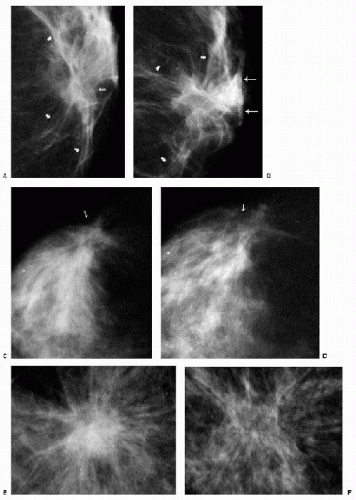
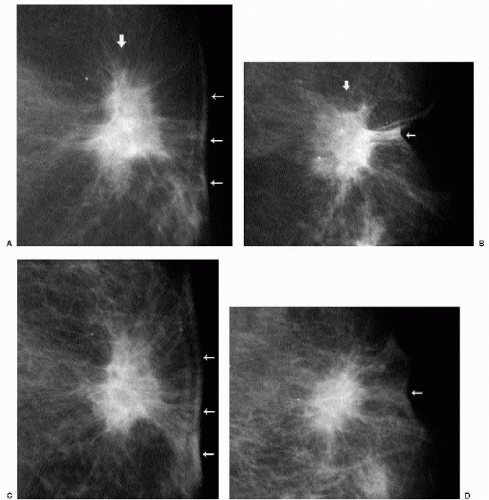
Changes that may be seen on a mammogram correlating with excisional biopsy sites include skin thickening, parenchymal distortion (Figure 10.2A-D), asymmetry, and a spiculated (Figure 10.2 E, F), round or oval mass. Except for parenchymal asymmetry, resulting from the removal of tissue from one breast, these changes usually resolve completely, remain stable after the first year, or slowly evolve with time (Figure 10.3). Increasing amounts of fat, dispersion of the density, oil cyst formation, and the development of dystrophic calcifications are changes that can be seen on follow-up (1, 2, 3). Occasionally, retained fragments from the localization wire or foreign bodies (Figure 10.4) may be seen at prior biopsy sites.
If a postoperative finding does not resolve completely, stability or progressive resolution needs to be established on annual studies. This is particularly important in patients with a personal or significant family history of breast cancer or if, histologically, the biopsy yielded a high-risk lesion (atypical ductal hyperplasia, lobular neoplasia, and multiple peripheral papillomas). In patients with a high-risk lesion diagnosed on excisional breast biopsy, we recommend a mammogram 6 months after the biopsy to establish the presence of any biopsy change (e.g., a new baseline for the patient). Changes seen 6 months after biopsy are unlikely to represent recurrent or interval cancer. On subsequent studies, we
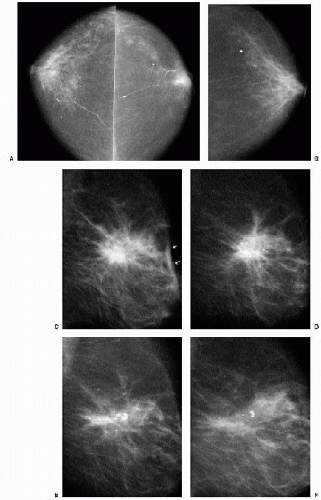
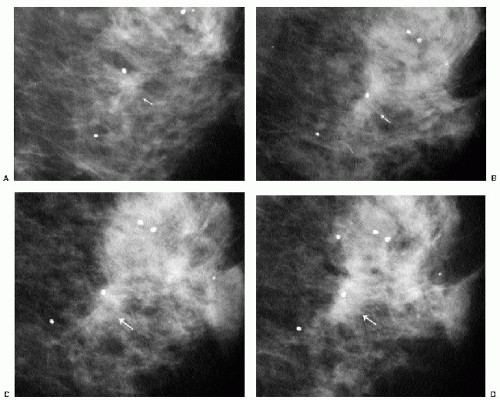
expect these changes to remain stable or, more commonly, evolve, becoming less prominent with time. Increases in distortion or density at a biopsy site after the initial 6-month postoperative study warrant a biopsy recommendation and a review of the original pathology. As yearly studies accrue, comparison is made to the earliest postoperative study available. Subtle progressive changes may not be readily apparent from year to year but can be quite obvious when comparison is made to the earliest available postoperative study (Figure 10.5).
expect these changes to remain stable or, more commonly, evolve, becoming less prominent with time. Increases in distortion or density at a biopsy site after the initial 6-month postoperative study warrant a biopsy recommendation and a review of the original pathology. As yearly studies accrue, comparison is made to the earliest postoperative study available. Subtle progressive changes may not be readily apparent from year to year but can be quite obvious when comparison is made to the earliest available postoperative study (Figure 10.5).
Postoperative changes remain stable or resolve progressively on annual studies. Repeat biopsy should be considered if there is increasing distortion or density at a prior biopsy site.
VACUUM-ASSISTED IMAGING-GUIDED BIOPSY
Vacuum-assisted imaging-guided breast biopsies with an 11-gauge (less commonly, 14-gauge) needle can result in complete removal of small lesions. In these patients, a metallic (titanium) clip is deployed to mark the site of the lesion. If a malignancy is diagnosed, the metallic clip is used to guide a preoperative wire localization so that a wide excision of the tumor site can be accomplished. These imaging-guided procedures, however, do not produce long-term changes in the mammographic appearance of the breast tissue. When a metallic clip is deployed at the time of the biopsy, and the patient does not undergo surgery, the clip is seen on follow-up studies. The clip usually remains, at or close, to the biopsy site; however, migration of the clip has been reported (4,5).
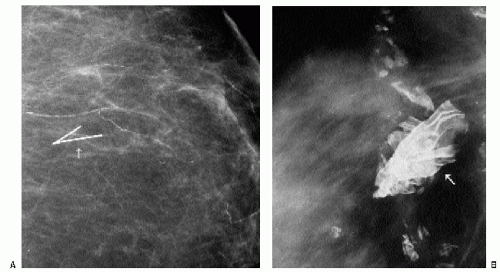
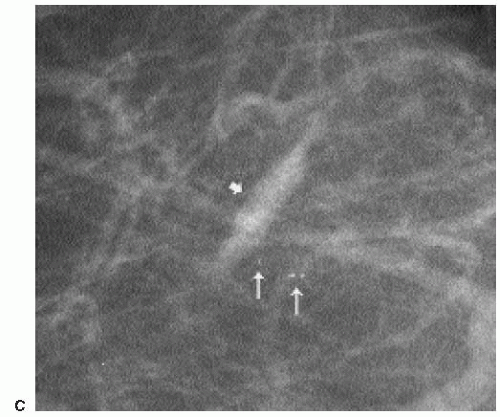
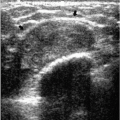
At the completion of the biopsy procedure, orthogonal images are obtained to document the location of the titanium clip. On these images, one or more air locules may be seen at the biopsy site with an associated area of increased density (Figure 10.6). The air and density are usually absorbed within the first several days after the biopsy. Rarely, increased density may be seen at the biopsy site secondary to hematoma formation. In some patients, this takes a tubular appearance, suggesting it is occurring along the needle track (Figure 10.7).
TRAUMA
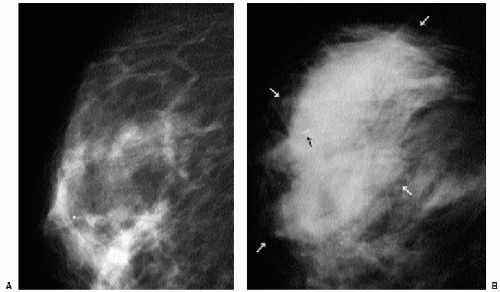
After trauma to the breast, patients may present acutely with an ecchymosis at the site of the trauma. Irregular areas of increased density, or mixed-density masses reflecting the presence of a hematoma and fat necrosis are seen mammographically (Figure 10.8). In some patients, parenchymal asymmetry or focal prominence of the trabecular markings is seen (Figure 10.9). Hyperechoic areas with associated hypoechoic to anechoic areas are commonly seen on ultrasound (Chapters 4 and 6). On follow-up, the ecchymosis resolves. Mammographic and ultrasonographic findings usually resolve completely; some patients develop a mass that is hard and palpable on physical exam, with a mixed-density lesion seen mammographically. Alternatively, an oil cyst and dystrophic calcifications may develop at the site of trauma.
After a seat-belt injury, patients may present with a bandlike area of increased density corresponding to the course of the belt. The findings are localized to the upper inner or central quadrants of the left breast or the lower inner quadrant of the right breast when the patient is the driver. Findings in the upper inner quadrant of the right breast or lower inner quadrant of the left breast may be identified when the patient is the front-seat passenger (6).
CONSERVATIVE BREAST CANCER TREATMENT
The primary aim of breast-conserving treatment is adequate local control of breast cancer. Wide surgical margins are desired in minimizing local recurrences. The cosmetic results, however, are an important secondary consideration. If a substantial amount of tissue needs to be removed to obtain clear margins in a patient with a small breast, cosmesis may not be acceptable. Radiation therapy is used to control any residual occult breast cancer. It is begun 2 to 5 weeks after the lumpectomy. Treatment is given 5 days a week for a total of
5 weeks and delivers 45 to 50 Gy of radiation to the affected breast. A boost to the lumpectomy site may be given using an electron beam or iridium implant, increasing the total dose delivered to 60 to 66 Gy (2).
5 weeks and delivers 45 to 50 Gy of radiation to the affected breast. A boost to the lumpectomy site may be given using an electron beam or iridium implant, increasing the total dose delivered to 60 to 66 Gy (2).
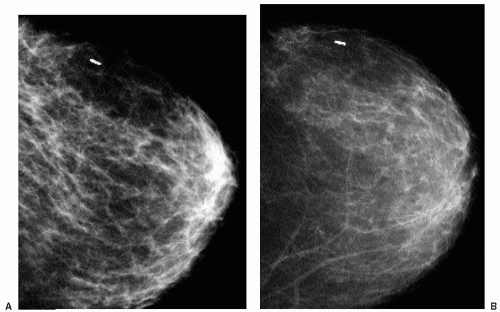
Mammographic changes after lumpectomy and radiation therapy are variable and usually evolve with time. Present acutely, edema is manifested by skin and trabecular thickening leading to diffuse increases in parenchymal density and reduced breast compressibility; it usually resolves within the first 2 years after treatment (Figure 10.10). Technical factors for adequate exposure of the remaining breast tissue need to be adjusted accordingly. Ultrasonographic changes in the acute setting include skin thickening, dilated lymphatics, and increased tissue echogenicity (Chapter 4). Residual skin thickening is seen in about 20% of women 2 years after radiation therapy (2).
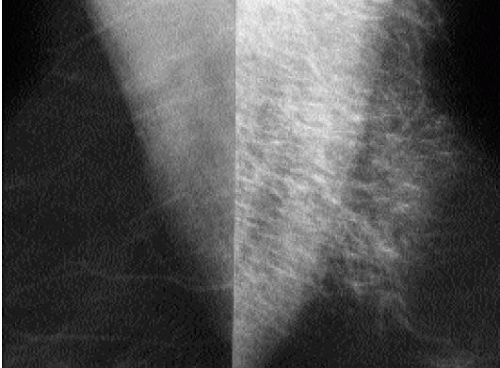 Figure 10.9 Hematoma. Thickening and increased density of the trabecular markings at the site of an ecchymosis in the upper portion of the left breast after trauma to the breast. |
Fat necrosis at the lumpectomy site is variably characterized by a spiculated mass or distortion (Figure 10.11). These changes contract and may resolve completely with time. As the spiculated mass or area of distortion resolves, oil cysts or dystrophic calcifications (Figure 10.12) can be seen developing at the lumpectomy site in some patients (2,7, 8, 9, 10, 11, 12).
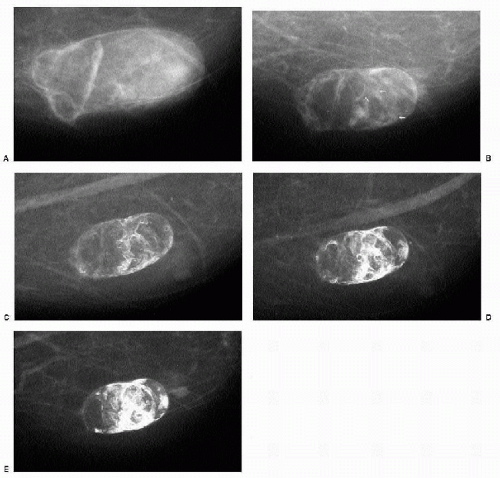
Fluid collections are seen in as many as half of patients within the first 4 weeks after the lumpectomy (2). Oval or round (Figure 10.13), well-circumscribed to ill-defined (Figure 10.14) or spiculated masses (Figure 10.15) at the lumpectomy site are variable in density and may have lucent locules or fluid-fluid levels. An internal halo partially outlining the inner margin may be present (Figure 10.16). Ultrasonographically, complex cystic masses that are predominantly cystic with septations, thickened walls, or echogenic nodules are imaged corresponding to the masses seen mammographically. Less commonly, a complex cystic mass that is predominantly solid with cystic spaces can be seen (Figure 10.17). Most fluid collections resolve within the first 2 years after the lumpectomy; however, some may persist for years. If the patient is asymptomatic, aspiration is not indicated. Reaccumulation of fluid is common after percutaneous drainage, and chronic draining sinuses can develop after aspirations (Figure 10.18). Draining sinuses can be hard to manage and often affect a patient’s quality of life significantly.
Currently, there is no consensus on an appropriate follow-up protocol for patients after lumpectomy. Some facilities recommend 6-month follow-ups of the treated breast for 3, 5, or 7 years and annual imaging of the contralateral, presumably normal breast (2). We obtain a preirradiation mammogram in patients who presented with an extensive area of calcifications. Rarely, we identify patients with residual calcifications at the lumpectomy site (9). These patients may benefit from reexcision before radiation therapy is started. After completion of radiation therapy, we recommend annual bilateral diagnostic studies (9). We do not routinely obtain 6-month follow-ups of the treated breast.
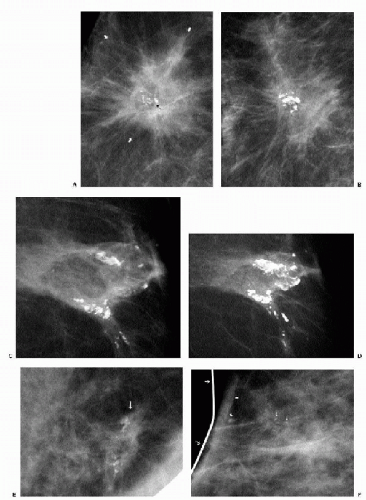
After conservative breast cancer surgery, patients are scheduled annually for diagnostic studies. In addition to routine views, we obtain a double spot compression magnification view of the lumpectomy site in tangent to the x-ray beam. When evaluating these patients, it is helpful to have information on those features of the initial tumor that may influence the likelihood of recurrence, including tumor size and grade, proximity of tumor to margins, presence of an extensive intraductal component, lymphovascular space involvement, and lymph node status. Additionally, it is helpful to know whether the patient had radiation treatment or chemotherapy and if she is being treated with tamoxifen. Ideally, the patient’s imaging evaluation at the time of diagnosis is available for review because recurrences often resemble the appearance of the primary tumor. The likelihood of recurrence is low in the first 2 years after treatment; it may be that some of the lesions identified within this time period reflect residual (inadequately treated) disease and not necessarily a recurrence. In the first 7 years after treatment, recurrences are likely to arise at or close to the lumpectomy site; after this, recurrences or second primary lesions develop with an equal frequency anywhere in the breast (not limited to the lumpectomy site) (13, 14, 15). The development of clustered, pleomorphic, linear or linearly oriented, round, punctate or amorphous (Figure 5.47 in Chapter 5) calcifications at prior lumpectomy sites should warrant biopsy (Figure 10.19). Developing masses (Figure 10.20) and increases in size and the overall density of areas of architectural distortion may also indicate recurrence. Rarely, recurrences present with diffuse changes (Figure 10.21).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree