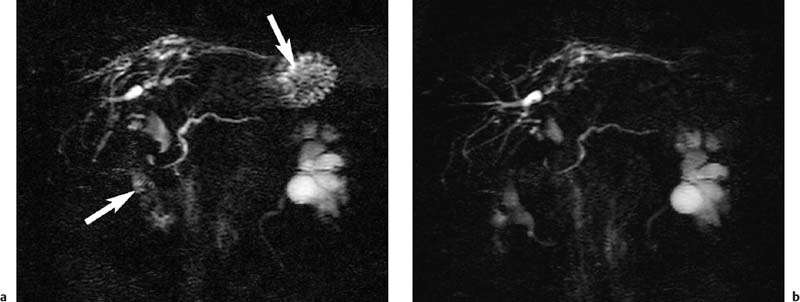
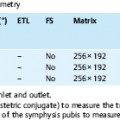
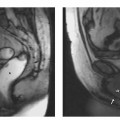
2 The Bile and Pancreatic Ducts Magnetic resonance cholangiopancreatography (MRCP) has evolved into an important noninvasive imaging modality for routine clinical evaluation of the pancreatobiliary tree. MRCP is a very elegant imaging tool, as it is fast and straightforward and has very few adverse effects. While invasive visualization of the bile ducts and pancreatic duct using conventional radiographic techniques requires intravenous injection of a biliary contrast medium (cholangiography) or direct instillation of contrast medium into the ducts (percutaneous transhepatic cholangiography [PTC] or endoscopic retrograde cholangiopancreatography [ERCP]), noninvasive MRCP exploits the very long T2 relaxation times of fluids for visualization of the ductal system. Conventional invasive procedures carry considerable risks, including the potential adverse effects of contrast administration. They are examiner-dependent and stressful for the patient. The most feared complications of PTC include bleeding and injuries to the bile ducts, while ERCP may cause duodenal perforation, pancreatitis,1,2 cholangitis, and duct perforation. Complication rates between 4 % and 11 % have been reported for ERCP.2,3 Another major advantage of MRCP is that it also provides more comprehensive diagnostic information on adjacent anatomic structures and organs, thus contributing to an earlier diagnosis. ERCP, on the other hand, is not only a diagnostic tool but, more importantly, can also be used to perform therapeutic interventions in the same session. Noninvasive MRCP and invasive ERCP therefore supplement each other and together are a mainstay of patient care. A comparative cost-benefit analysis of ERCP and MRCP concludes that primary diagnosis with MRCP is economically advantageous,4 which is an important consideration for health-care providers in the era of cost cutting and reimbursement based on diagnosis-related groups (DRG). Noninvasive MRCP of the pancreatobiliary tree can contribute valuable diagnostic information in patients with suspected involvement of the bile ducts or pancreatic duct if the clinical symptoms and the first-line imaging test (ultrasound) do not yield a definitive diagnosis. This is primarily the case in patients with suspected biliary pancreatitis, acute or chronic cholangitis, strictures or anomalies of the bile ducts (e. g., Caroli disease, choledochocele, biliary atresia), anatomic variants (pancreas divisum), and cholangiocarcinoma (e. g., Klatskin tumor). In patients scheduled for an interventional procedure, preinterventional localization of strictures and stones and detection of pseudocysts are crucial for planning the approach. Imaging provides the clinician with detailed information on the anatomic situation, thereby facilitating interventions such as laparoscopic surgery. High spatial resolution is crucial for detailed anatomic visualization of the target structures (very small bile ducts, side branches of the pancreatic duct), which is not achieved with the integrated whole-body resonator. Surface coils have the essential advantage of providing a better signal-to-noise ratio (SNR), which improves spatial resolution and contrast while also shortening imaging time (breath-hold sequences, see below). However, the most suitable coil is a multielement body or torso phased-array surface coil (usually consisting of 4–8 elements, which, in addition to the aforementioned advantages, enables use of a larger field of view (FOV) and reduces artifacts. When a multielement coil is used, the entire biliary tree and pancreatic duct as well as the liver and pancreas can be imaged in a single session without coil repositioning. Conventional imaging of the liver and pancreas should be part of any routine MRCP protocol.5 MRCP exploits the long T2 relaxation time of fluids (several seconds for bile) for indirect visualization of ductal structures. At the same time, signal from other tissue structures is effectively suppressed when heavily T2-weighted sequences are used because the echo time (TE) of these sequences is a multiple of the T2 relaxation time of stationary tissues (40–100 ms in the abdomen). However, there may be overlap from other fluid-containing structures such as cysts (pancreatic pseudocysts, hepatic and renal cysts), intestinal loops, and the renal collecting system and ureter. Sequences based on the rapid acquisition with relaxation enhancement (RARE) technique,6 such as the half-Fourier acquisition single-shot turbo spin echo (HASTE) sequence, are among the most widely used sequences for MRCP. The examination can be performed in one of two complementary ways: acquisition of a single thick-slab projection image and 2D acquisition of a series of thin slices with subsequent reconstruction using the MIP (maximum intensity projection) algorithm.7 While thick-slab imaging (which takes < 6 s) is well tolerated during breath-hold, multislice acquisition requires a breath-hold of ca. 20 s, which is beyond the capacity of some patients. Alternatively, MRCP can be performed with the patient breathing freely if acquisition is synchronized with the respiratory cycle. Various techniques are available from different vendors, but the equipment is cumbersome to handle and has found little acceptance. A practical, software-based alternative is respiratory triggering using the navigator echo approach. The navigator technique measures the position of the diaphragm to synchronize acquisition accordingly (PACE technique: prospective acquisition correction – Siemens) and was originally developed for cardiac imaging. The main advantage of respiratory navigator triggering is that the longer acquisition time allows use of sequences with much better spatial resolution.8 Oral Contrast Media When heavily T2w thick-slab images are acquired at various projections along the course of the pancreatic and biliary ducts, there will invariably be some overlap from other fluid-filled structures, particularly the stomach and duodenum. The signal from gastrointestinal fluids can be suppressed by administering a negative oral contrast medium containing iron oxide particles. Since such contrast media do not distend the ducts, normal anatomy is not distorted.9 However, one must be aware that negative oral contrast media will also obscure anatomic landmarks such as the descending duodenum (Fig. 2.1). There is controversy in the literature as to whether the diagnostic quality of MRCP can be improved most effectively by suppressing or enhancing the gastrointestinal (GI) signal. Positive oral contrast media cause the GI lumen to appear bright, thereby improving delineation of the liver and pancreas from intestinal loops if MRCP is supplemented by conventional axial MRI of these two organs. The latter is desirable in all patients undergoing MRCP because it will identify possible external causes of ductal narrowing as well as other pathology not affecting the ducts (see below). Finally, the decision whether or not to use oral contrast medium is also affected by increasing pressure to reduce health-care costs. In this context, blueberry juice, which has the same effects on T2w images as negative oral contrast medium, has been proposed as an inexpensive alternative.10 Some investigators dispense with oral contrast administration altogether; in this case, MRCP should be performed in the morning after fasting when only a small amount of fluid is present in the upper GI tract. Other Medication Motion artifacts due to respiration and upper GI tract peristalsis are a major problem for MRCP, given the small size of some of the ductal structures. Most patients can hold their breath for thick-slab imaging, which has an acquisition time of less than 10 s. Peristalsis should be suppressed by intramuscular injection of an antispasmodic at the beginning of the examination unless a patient has contraindications. Visualization of the pancreatic ductal system (especially side branches and the accessory duct) can be improved by secretin stimulation. Intravenous secretin injection enhances pancreatic exocrine function, which improves filling of the ducts and thus allows more accurate evaluation of ductal morphology. In addition, pancreatic exocrine function can be assessed by quantitative determination of subsequent duodenal filling.11,12 Imaging 5 min after intravenous injection yields the best results. Secretin stimulation is contraindicated in patients with acute pancreatitis or acute on chronic pancreatitis. General Preparation MRCP is best performed in the morning when secretion in the upper GI tract is minimal. Patients should fast for at least 6 h and abstain from even small amounts of fluid before the examination. Oral Contrast Media We usually perform MRCP with an oral contrast medium solution, which the patient starts drinking approximately 30 min before the examination (at least 300 mL; 600 mL creates optimal imaging conditions). The contrast solution can be mixed 1:1 with water to prevent susceptibility artifacts that would result from sedimentation of iron oxide particles in the immobilized GI tract. Imaging of Upper Abdominal Organs An MRCP protocol should comprise conventional plain MRI of the liver and pancreas. If these images reveal a lesion requiring further characterization after intravenous contrast administration (Gd-based, low-molecular-weight contrast medium), MRCP is followed by a dynamic contrast-enhanced MRI study of the respective organ (for further details see the chapter on the liver or pancreas). Intravenous contrast media do not impair the quality of the MRCP examination, and their T2-shortening effect even reduces interference from overlying contrast-enhancing structures on thick-slab images, especially the renal collecting systems and ureters. MRCP of the Bile and Pancreatic Ducts The MRCP sequences we use are summarized in Table 2.1. The examination begins with acquisition of a fast axial T2w multislice sequence (e. g., T2w HASTE) that covers the entire upper abdomen and is used to plan the subsequent MRCP sequences. Next, a coronal thick-slab projection image (120 mm) is acquired with a fast, fat-saturated T2w sequence and a FOV adapted to the imaging plane. A single projection image acquired in this way will depict the central bile ducts and most of the pancreatic duct (Fig. 2.2a). Such an image has a low SNR but nevertheless provides a good anatomic overview of the biliary and pancreatic duct systems. Additional thick-slab acquisitions are then performed with projections and slice thicknesses (30–80 mm) selected according to the duct segments imaged. Optimal orientations for targeted visualization of specific parts of the ducts depend on the patient’s anatomy, which is why only some very general suggestions can be made here (in terms of a clockface): parallel to the pancreatic duct (projection between 8 and 9 o’clock); parallel to the common bile duct (projection at approximately 10 o’clock); parallel to the right and left hepatic ducts (highly variable). Additional imaging planes may be acquired as deemed necessary depending on the presence of normal anatomic variants and site of pathology (Fig. 2.2b–d). Thick-slab imaging is followed by multislice, thin-slice acquisitions (3 mm, no interslice gap) for evaluating very small ductal structures. The individual series of thin slices are acquired at the same angles as the corresponding thick-slab images. The acquisition time for one such series is ca. 20–23 s. We perform thin-slice MRCP with a T2w multislice HASTE sequence (Fig. 2.3a–c
P. Asbach and H.B. Gehl
Introduction
Indications
Imaging Technique
Coils
Pulse Sequences
Patient Preparation
Recommended Imaging Protocol
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree