The carpal tunnel is a fibro-osseous tunnel along the volar aspect of the wrist. Understanding the normal anatomy, variant anatomy, and pathology of the carpal tunnel is important in evaluating the patient with carpal tunnel syndrome. Carpal tunnel syndrome is the most common entrapment neuropathy of the upper extremity. It is defined as impairment of the motor and/or sensory function of the median nerve as it transgresses the carpal tunnel. This syndrome occurs most commonly in patients who perform repetitive motions. Numerous other etiologies are also recognized, including acute trauma, infection, mass lesions, variant anatomy, infiltrative disorders, and intrinsic nerve abnormalities. In many cases, a combination of factors is responsible.
Carpal tunnel syndrome is usually diagnosed by its characteristic clinical and electrophysiological features. Currently, the role of imaging in carpal tunnel syndrome is not clearly defined. Sonography or MRI may be helpful in patients with a confusing clinical presentation or nonresponsiveness to conservative treatment. There may be a need to confirm diagnosis in cases in which nerve conduction studies are equivocal. A firm diagnosis is important when endoscopic surgical techniques are used. This procedure is minimally invasive, but the surgeon does not directly visualize the contents of the carpal tunnel. Preoperative imaging can help establish the diagnosis in this scenario. Pathology in the carpal tunnel will often present with carpal tunnel syndrome that is refractory to conservative or surgical therapy. Imaging can characterize tumors, anatomic variants, and tenosynovitis in this region and assist in surgical planning.
Anatomy
Median Nerve
The median nerve may be compressed in many locations along its path from the brachial plexus to the carpal tunnel. Knowledge of its branches and innervation patterns assists the clinician and the radiologist in forming an imaging strategy to demonstrate the level of pathology in difficult cases. The median nerve arises from the lateral and posterior cords (C6–T1) of the brachial plexus and travels down the medial aspect of the upper arm. It enters the forearm between the humeral and ulnar heads of the pronator teres muscle. In the proximal forearm, the median nerve provides motor supply to the pronator teres, flexor carpi radialis, flexor digitorum superficialis, and the palmaris longus. Small branches also innervate the elbow at this level. The main branch arising from the median nerve distal to the elbow is the anterior interosseous nerve. This is a purely motor nerve that supplies the pronator quadratus, the flexor pollicis longus, and portions of the flexor digitorum profundus.
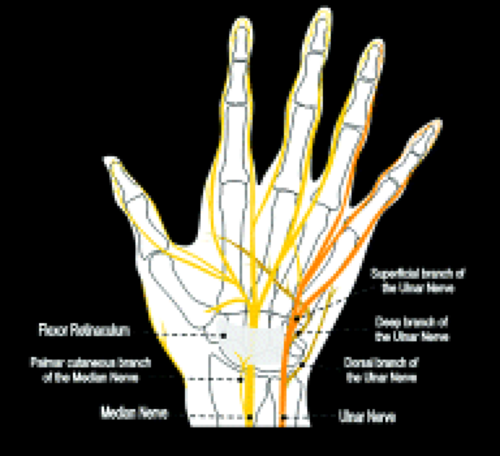
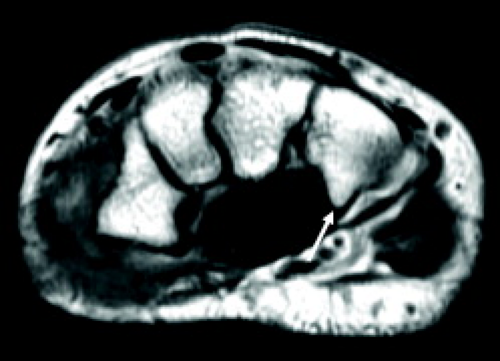
The median nerve continues distally within the forearm between the flexor digitorum superficialis and profundus. A palmar cutaneous branch arises just before the median nerve enters the carpal tunnel (Fig. 16.1). This sensory branch travels adjacent to the flexor carpi radialis and supplies sensation to the radial aspect of the palm and the thenar eminence. The median nerve distal to the carpal tunnel provides motor supply to the thenar eminence, the superficial part of the flexor pollicis brevis as well as the first and second lumbricals. Terminal sensory branches supply the volar surface of the lateral three and a half digits.
Median nerve dysfunction at the level of the carpal tunnel results in a characteristic clinical pattern. The motor function of the wrist flexors is unaffected because they are supplied by proximal branches of the median nerve and the anterior interosseous nerve. Similarly, the palmar cutaneous branch of the median nerve arises proximal to the carpal tunnel and sensation to the radial aspect of the palm is maintained. The motor branches to the thenar eminence and sensation to the palmar surface of the lateral digits are affected by median nerve dysfunction at this level.
Carpal Tunnel
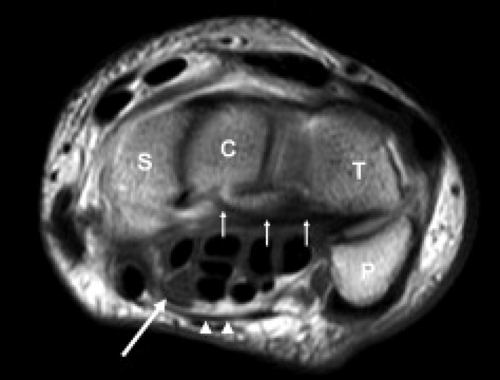
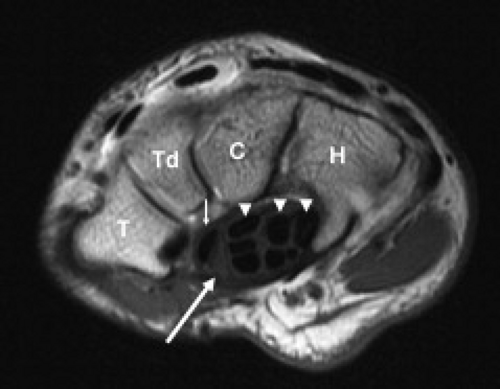
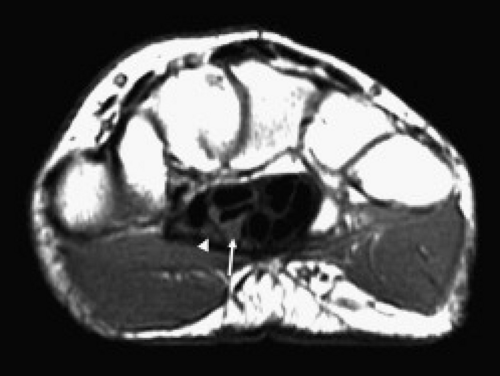
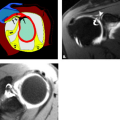
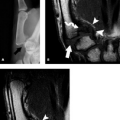
The carpal tunnel begins at the level of the pisiform and the tubercle of the scaphoid. The dorsal aspect of the proximal carpal tunnel is formed by the distal scaphoid, proximal capitate, trapezium, and pisiform (Fig. 16.2). The carpus forms a concave contour with respect to the tunnel on transverse images and is covered along its volar aspect by the extrinsic ligaments of the wrist. These ligaments include the palmar lunotriquetral and palmar scaphotriquetral proximally and the capitate–scaphoid and the capitate–trapezium slightly more distally. The carpus and extrinsic ligaments are often described as the floor of the carpal tunnel. The tunnel continues to the level of the hamate and the trapezium. The distal carpal row demonstrates a similar concave contour formed by the body of the hamate, capitate, the trapezoid, and the trapezium (Fig. 16.3). A small fat plane, known as Parona’s fat pad, develops just volar to the capitate–trapezium ligament adjacent to the distal carpal row at this level. The size of this fat plane is variable.
The flexor retinaculum, also known as the transverse carpal ligament, encloses the carpal tunnel along its volar surface. It attaches to the pisiform and the tubercle of the scaphoid proximally and the hamulus of the hamate and the ridge of the trapezium distally. The thenar musculature arises from the flexor retinaculum and the trapezium. The hook of the hamate and flexor retinaculum provide the attachments for the hypothenar muscles.
The flexor retinaculum extends anteriorly and is continuous with the palmar aponeurosis.
The flexor retinaculum extends anteriorly and is continuous with the palmar aponeurosis.
The contents of the carpal tunnel include the tendons from the flexors of the fingers and the thumb as well as neurovascular structures. The eight tendons of the flexor digitorum profundus (FDP) and flexor digitorum superficialis (FDS) are invested in a common synovial sheath. The most volar tendons are those arising from the FDS that extend to the third and fourth digits. Just deep to this are the superficial tendons that attach to the second and fifth digits. The tendons of the FDP are found at the floor of the carpal tunnel (Fig. 16.2). The flexor pollicis longus tendon has its own tendon sheath and is located just radial to the other flexor tendons within the carpal tunnel. These tendons arise from muscle bellies proximal to the carpal tunnel. If muscles are seen within the carpal tunnel, variant anatomy should be suspected.
The median nerve position in the carpal tunnel is variable when the wrist is in neutral alignment. It is generally found anterior to the FDS tendon of the index finger or laterally between this tendon and the flexor pollicis tendon (Fig. 16.2) (1). The median nerve changes position relative to the adjacent tendons on both flexion and extension. In extension, the median nerve usually moves anterior to the index finger FDS tendon, touching the flexor retinaculum. In the flexed position, the median nerve can usually be found between the FDS of the index finger and the flexor pollicis longus. It may extend into a more volar position between the FDS tendons supplying the third and fourth digits. The shape of the median nerve is also variable. It normally has an ovoid shape when the wrist is in neutral position and on extension. Some flattening of the median nerve is seen in asymptomatic patients on flexion.
Anatomic Variants
Anatomic variants are common in the wrist. Many of these variants occur with increased frequency in patients with carpal tunnel syndrome and have been implicated in its pathogenesis. Singer et al (2) described anatomic variants in 41% of 137 patients surgically treated for carpal tunnel syndrome. These anatomic variants include variant anatomy of normally present
muscles and the median nerve. Variants can also occur with structures not normally found in the wrist such as a persistent median artery and anomalous musculature.
muscles and the median nerve. Variants can also occur with structures not normally found in the wrist such as a persistent median artery and anomalous musculature.
Median Nerve
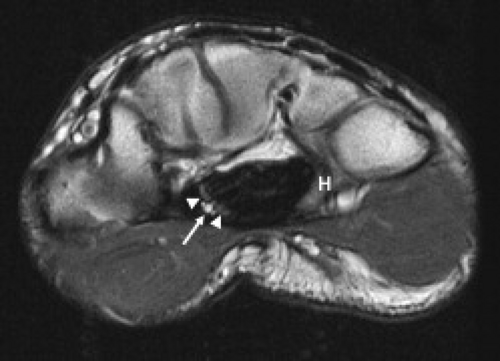
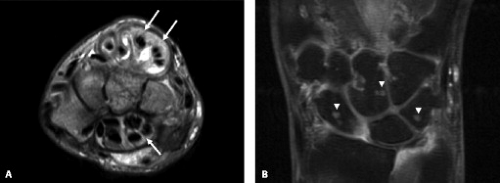
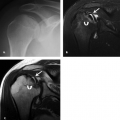
Variants of the median nerve are not associated with increased risk of carpal tunnel syndrome. However, the presence of unusual anatomy may complicate the diagnosis from both a clinical and imaging perspective. Median nerve variants include accessory branches arising before the carpal tunnel, accessory branches arising in the distal carpal tunnel, a different course of the thenar branch, and a bifid median nerve (2). The most common variant is a bifid median nerve, which occurs with prevalence approximately 3% of the time (3). This variant has been demonstrated on both ultrasound and MRI (4) (Fig. 16.4). It is important to recognize a bifid median nerve on imaging. The established criteria for abnormal enlargement of the median nerve cannot be used to help diagnose carpal tunnel syndrome in this scenario.
Median Artery
A persistent median artery has a reported prevalence between 5% and 23% (5, 6). A bifid median nerve or early bifurcation of the median nerve within the carpal tunnel is commonly associated. The median artery is usually approximately 1 mm in diameter and generally asymptomatic (6). However, carpal tunnel syndrome has been reported in patients with a median artery greater than 3 mm (6) or in patients who present with thrombosis of the median artery. A median artery is easily visualized with both ultrasound and MRI (Fig. 16.4). The persistent median artery provides a significant contribution to the palmar arch in 4% to 5% of cases. Therefore, treatment with resection of the flexor retinaculum and preservation of the median nerve have been advocated and shown to be an effective treatment (7).
Osseous Structures
Variation in the size of the carpal tunnel has been proposed as an etiologic factor in carpal tunnel syndrome. A decreased volume of the carpal tunnel has been demonstrated in patients with carpal tunnel syndrome in early studies (8). These studies were performed on early computed tomography scanners and could not visualize the transverse carpal ligament. Exclusively osseous landmarks were used to calculate carpal tunnel volume. Later studies that included the flexor retinaculum as part of the volume calculation did not show a significant difference in the carpal tunnel volume in symptomatic patients (9).
Variations of the hook of the hamate include bipartite, hypoplastic, and aplastic hooks of the hamate. Several authors have suggested these variants play a causative role in carpal tunnel syndrome. Variants of the hook are found in 0.1% to 0.8% of asymptomatic patients (10, 11) compared with 2.3% to 3.0% of symptomatic patients (10, 12).
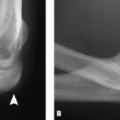
The smaller hamulus represents a lower attachment site for the transverse carpal ligament and thus reduces the volume within the carpal tunnel. Variations in this region are of further importance because surgeons use the hook of the hamate as an anatomic landmark for endoscopic carpal tunnel release (13). Preoperative carpal tunnel views have been advocated to assist in the identification of these variants that may make endoscopic release more challenging (12). A bipartite hook of the hamate is defined as a smooth radiolucent line dividing the hamate hook into two segments. Chow et al. (10) describes a hook of hamate index in which the length of the hook is divided by its width at the base. A normal hook of hamate index was found to be 1.7 (standard deviation, 0.24) on hook–of-hamate views. Hypoplastic and aplastic hooks of the hamate were defined as having an index of 1.3 and 0.6, respectively (Fig. 16.5).
Anomalous Musculature
Anomalous musculature resulting in carpal tunnel syndrome can be subdivided into anomalous muscles or expected muscles with variant anatomy. It is important to note that although the prevalence of anomalous muscles of the wrist is not unusual (2, 14), they are an uncommon cause of compression neuropathy within the carpal tunnel and may be an incidental finding. In a study based on 681 surgical cases of carpal tunnel syndrome, anomalous musculature was the proven causative agent in 1.5% of cases (15). In another study based on 147 consecutive patients surgically treated for carpal tunnel syndrome, 41% of patients demonstrated variant anatomy (2). The authors made an extended open incision to better visualize the musculature. Significant increased prevalence of variant anatomy was found in younger patients. Most authors postulate that it is not the existence of the variant anatomy that produces symptoms, but rather the hypertrophy of it secondary to repetitive activity (16–17).
The muscle–tendon unit most likely to produce neuropathy at the level of the carpal tunnel is the palmaris longus. It accounts for approximately half of the reported cases of muscle anomalies related to median compression neuropathy (18). The palmaris longus tendon is a wrist flexor that demonstrates markedly variable anatomy. Classically, it arises from the medial epicondyle and terminates in a long, slender tendon that travels medial to the flexor carpi radialis. It travels superficial to the carpal tunnel and inserts into the palmar aponeurosis. It is absent in 16% of the population. It is markedly variable and may consist purely of tendon or entirely of muscle from origin to insertion. It may also demonstrate a reversed structure with a tendinous origin and a distal muscle belly (19, 20). Effort-related median nerve compression has been attributed to a reversed palmaris longus muscle (21). Anomalous insertion of the palmaris longus with resultant carpal tunnel syndrome has also been reported (22). Resection of a reversed, hypertrophied palmaris longus muscle has been described with relief of symptoms and resolution of nerve conduction abnormalities (23). An accessory muscle attaching to the palmaris longus tendon and extending to the hypothenar eminence was found in 8% of patients with surgically treated carpal tunnel syndrome (2).
Variations in the lumbrical muscles represent the second most common muscle variation in muscles implicated in carpal tunnel syndrome. The lumbricals are intrinsic muscles of the hand that arise from the tendons of the FDP. They continue along the radial side of the digit and insert into the extensor digitorum expansion. The site of origin of the lumbrical muscles is highly variable. In most individuals, they insert on the FDP tendons after they exit the carpal tunnel. Numerous reports of lumbricals arising from the forearm in patients with carpal tunnel syndrome can be found in the literature (24–26). Imaging technique is important to avoid an incorrect diagnosis of aberrant lumbrical insertions. The wrist should be imaged with the fingers in extension. Flexion at the metacarpal and proximal interphalangeal joints may result in normal lumbrical incursion within the carpal tunnel, simulating aberrant origins.
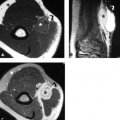
Low-lying muscle bellies of the FDS (Fig. 16.6) and flexor pollicis longus also result in crowding in the carpal tunnel (27, 28). Numerous reports of carpal tunnel syndrome in patients with accessory musculature have been published (2, 29–33). These anomalous muscles include the palmaris profundus and accessory FDP. An anomalous transverse muscle just superficial to the transverse ligament with attachments to the thenar and hypothenar eminences has also been implicated in carpal tunnel syndrome (29, 34).
Carpal Tunnel Syndrome
Epidemiology
Carpal tunnel syndrome is the most common compressive neuropathy of the upper extremity. In most studies, the prevalence rate is measured between 2% and 5%. In one large study of 2466 patients, 94 (3.8%) persons met clinical diagnostic criteria for carpal tunnel syndrome (35). Sixty-six (2.7%) of those who met clinical criteria had positive electrodiagnostic findings.
Carpal tunnel syndrome is found more commonly in women than men. Among older patients, the incidence is four times more common in women than men. This sex difference is generally smaller in younger populations. An age of presentation at 30 to 60 years old is typical. The dominant hand is usually affected, although a significant number of cases are bilateral with reported rates varying from 10% to 50% (36). This reduces the usefulness of comparison of the other side because nerve abnormalities may occur before the onset of symptoms. Carpal tunnel syndrome occurs with an increased incidence in those with occupations or interests that involve repetitive movement at the wrist, including meat packers, frozen food processors, musicians, dental hygienists, and radiologists (37). Occupations necessitating
excessive force with the hand or excessive wrist flexion and extension are particularly at risk (35).
excessive force with the hand or excessive wrist flexion and extension are particularly at risk (35).
Clinical Presentation
The classic clinical presentation of carpal tunnel syndrome is pain and dysesthesias in the distribution of the median nerve. Patients often experience transient burning pain and numbness, which is worse at night. The digits affected are the thumb, index, and middle and radial half of the fourth finger. Sensation of the palm and thenar eminence is usually unaffected, because the palmar cutaneous branch arises from the median nerve before the carpal tunnel.
Physical examination may demonstrate sensory findings from minimal hypesthesia to anesthesia in the palmar aspect of the lateral three and a half digits. Classic tests for carpal tunnel syndrome include a positive Tinel’s sign and positive Phalen’s test. A positive Tinel’s sign is present when percussion over the median nerve results in dysesthesia in a median nerve distribution. A positive Phalen’s test is when symptoms are elicited by flexion at the wrists opposing the dorsum of opposite wrists together. Tourniquet compression or direct compression also can elicit signs of median nerve entrapment in patients with carpal tunnel syndrome.
Motor weakness in the setting of the carpal tunnel syndrome may be subtle and transient. The median nerve distal to the carpal tunnel provides motor supply to the thenar eminence, the superficial part of the flexor pollicis brevis as well as the first and second lumbricals. Muscle atrophy of the thenar eminence and loss of function are late findings. The abductor pollicis longus may show early involvement because it is partially innervated by the median nerve. Overall, a clinical history and examination has been estimated to have sensitivity of 94% (38).
Electrophysiology
The most widely used diagnostic test for carpal tunnel syndrome is nerve conduction studies. Latency of the distal median nerve demonstrates very good accuracy in diagnosing carpal tunnel syndrome. The true accuracy is difficult to gauge because of lack of a gold standard. A patient with a characteristic clinical presentation or improvement with surgery is often used when evaluating electrodiagnostic studies. The false-negative rate has been reported at 10% to 15% (39, 40). These false-negatives may occur secondary to anomalous innervation patterns. In addition, symptoms are intermittent and may arise from small, unmyelinated fibers that are invisible to surface electrodes (41). Many reports describing patients who benefit from carpal tunnel surgery despite negative nerve conduction studies support the assertion that false-negative tests occur.
The false-positive rate is unknown because nerve conduction abnormalities often occur before the development of symptoms. The specificity is reported at 95% to 99%. This is based on the threshold of two standard deviations greater than the mean latency time in control groups. These control groups were generally comprised of young, healthy volunteers. Older patients may have longer conduction times and false-positives may occur in this subject group. In a study of 125 age-stratified asymptomatic subjects, electrophysiological median neuropathy was found in 23 (18%). Ten of these false-positives were found in patients older than 65 years (35). Other studies found positive tests in 9% to 16% of asymptomatic patients resulting in a positive predictive value of 30% (42). At any rate, the data suggest that there is an increased trend in false-positive studies in elderly patients. Age-adjusted electrophysiological criteria help to reduce false-positive studies but reduce sensitivity. Imaging may assist diagnosis in this population. Quantification of false-positive rates is further complicated by the possibility that these may be true-positives in asymptomatic patients. There may be a preclinical median nerve neuropathy in these patients.
Electrophysiological testing may be unreliable in patients with diabetes. No significant difference in nerve conduction study data was found when comparing patients with diabetes with (112) and without (311) symptoms of carpal syndrome (43). The authors recommended that therapeutic decisions in patients with clinical criteria for carpal tunnel syndrome be made independently from nerve conduction study findings. This may represent another subset of patients who would benefit from an imaging diagnosis of carpal tunnel syndrome.
False-positive diagnoses may also occur in patients with persistent symptoms after carpal tunnel release. Nerve conduction abnormalities may persistent for months or longer after successful retinaculum release (44).
In summary, electrodiagnostic tests are an inexpensive, accurate method of diagnosing carpal tunnel syndrome. Accuracy of nerve conduction studies may be decreased in patients with diabetes and elderly patients. MRI or sonography may be a helpful diagnostic tool in these subsets of patients or those in whom the clinical presentation and electrophysiological testing are discordant.
Pathophysiology
Compression neuropathy of the median nerve presents with the signs and symptoms of carpal tunnel syndrome. The final common pathway is increased interstitial fluid pressure (45) in the tissues within the carpal tunnel. The normal pressure in the carpal tunnel is 25 mmHg. Maximal pressures are reached with flexion of the wrist, typically around 32 mmHg (46). In patients with carpal tunnel, these pressure changes are more pronounced and pressures of 110 and 90 mmHg on flexion and extension, respectively, are typically found (37). This increased pressure results in transient ischemia with decreased perfusion of the median nerve. Intermittent perfusion of the cellular tissue between this episodic ischemia produces free radicals. With continued repetitive motion, the normal antioxidant response is overwhelmed and cellular injury ensues (47). Tissue injury and its response are seen in the flexor tenosynovium and the median nerve (48). Levels of prostaglandin E2 and interleukin-6 are found in rates five times that of asymptomatic volunteers, thought to be the result of recurrent ischemia and reperfusion injury.
Swelling of the median nerve is frequently seen proximal to the site of obstruction. This has been attributed
to an increase in the amount of endoneurial connective tissue, edema in the epineurium and endoneural space, and obstruction of axoplasmic flow. Late-stage intraneural fibrosis can occur in the median nerve and loss of function may become irreversible.
to an increase in the amount of endoneurial connective tissue, edema in the epineurium and endoneural space, and obstruction of axoplasmic flow. Late-stage intraneural fibrosis can occur in the median nerve and loss of function may become irreversible.
In general, abnormalities that increase pressure with the carpal tunnel arise from increased mass effect of the intrinsic components of the carpal tunnel or from extrinsic compression (49). Extrinsic masses include masses in the adjacent region, variant anatomy, and osseous abnormalities such as carpal instability or fracture. Increased pressure within the carpal tunnel can be caused by tenosynovitis, masses, increased fat deposition, edema, and anatomic variants.
Intrinsic causes of Carpal Tunnel Syndrome
Tendinopathy
The most common cause of carpal tunnel syndrome is flexor tendinosis or tenosynovitis resulting from repetitive wrist motion (50). Friction forces among the median nerve, tendons, and the transverse carpal ligament during flexion and extension occurs in this circumstance. Repetitive motion leads to tendon sheath synovial hypertrophy, tendinosis, and tendon enlargement with resultant increase in carpal tunnel pressure. MRI and sonography identify these changes commonly in symptomatic patients (51, 52). MRI demonstrates tendon changes, including tendon thickening and increased T1- and T2-weighted intrasubstance signal. Synovial hypertrophy of the tendon sheath is visualized and is usually isointense or hyperintense on T2-weighted images. Fluid within the tendon sheaths is present in severe cases.
Inflammatory tendinopathy and tenosynovitis may present as carpal tunnel syndrome. The differential for marked tendinopathy in the flexor compartment includes severe repetitive strain, rheumatoid arthritis, seronegative spondyloarthropathy, infections, gout, calcium pyrophosphate dihydrate deposition disease, amyloid, pigmented villonodular synovitis, hemophilia, and tumors arising from the synovium. These changes may be mild and simulate mechanical tendinopathy caused by repetitive motion. More commonly, these secondary causes of tendinopathy result in exuberant inflammation of the synovium of the tendon sheath and fluid within the tendon sheath. These changes are visualized on imaging and the presence of certain findings should suggest a secondary cause of tendinopathy. On MRI, the previously described changes in the tendons and tendon sheaths are usually more prominent. In addition, prominent enhancement of the peritendinous tissue is found with the addition of intravenous gadolinium. These changes are often not limited to the flexor compartment. MRI also demonstrates enhancing erosions in the adjacent carpus.
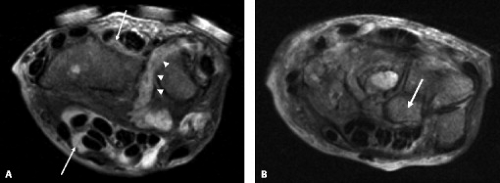
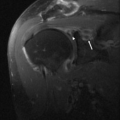
The most common presentation of tuberculous infection in the wrist is tenosynovitis. The flexor compartment is usually the main site of abnormality, although the extensor compartments are also frequently affected. Patients with tuberculous arthropathy may present with carpal tunnel syndrome (53). Spread to contiguous bone from the tendon sheath infection may occur. Although MRI often demonstrates nonspecific changes of tendonitis and tenosynovitis (Fig. 16.7), some characteristic findings may suggest the diagnosis. Synovial hypertrophy that is low signal on T2 may be present and suggests granulomatous involvement (54). Numerous small low-signal foci within the fluid are common in mycobacterial infection representing debris, caseous material, or rice bodies. Rice bodies were found in two of eight MRI studies of the wrist in a series of patients with proven tuberculosis infection (55). These are visualized as small, low T2-signal lesions within the hypertrophied synovium. Osseous erosions within the carpus may also refine the diagnosis. Tuberculous tenosynovitis may present as carpal tunnel syndrome. Three patients presented with
carpal tunnel syndrome in one series, all of which had MRI demonstrating encasement of the median nerve with enhancing tissue (55). Treatment with antibiotics is often attempted. However, in most cases, vigorous surgical débridement is necessary (56). MRI with gadolinium is useful in this clinical scenario by clearly depicting the extent of tenosynovial hypertrophy, assisting preoperative planning.
carpal tunnel syndrome in one series, all of which had MRI demonstrating encasement of the median nerve with enhancing tissue (55). Treatment with antibiotics is often attempted. However, in most cases, vigorous surgical débridement is necessary (56). MRI with gadolinium is useful in this clinical scenario by clearly depicting the extent of tenosynovial hypertrophy, assisting preoperative planning.
Fungal infection of the tendon sheaths is a rare cause of carpal tunnel syndrome. The most common etiologies include histoplasmosis and blastomycosis. Other fungi, including coccidioidomycosis, have been reported, especially in the setting of the immunocompromised patient. In a literature search of cases of tenosynovitis caused by histoplasmosis, six of 10 patients presented with carpal tunnel syndrome (57). This can be a difficult diagnosis to make. In the majority of these patients, no other evidence of infection was identified. Most of these patients had recurrent symptoms for months and the diagnosis was not suspected until it became apparent on surgical exploration.
Gout may cause carpal tunnel syndrome primarily by causing tenosynovitis of the flexor tendon sheath. Mass effect from gouty tophi within the carpal tunnel may contribute to symptoms. These tophi may occur anywhere, including the transverse ligament and median nerve (58). In a retrospective study in patients with suspected gout-related carpal tunnel syndrome (59), 18 of 20 MRI studies demonstrated tophi along the floor of the tunnel between the carpal bones and the flexor tendons. Nine patients demonstrated evidence of tophaceous deposits in the flexor tendons and tendon sheaths. In general, the tophaceous deposits were low signal on T1 and variable signal on T2-weighted images with avid enhancement. The tophi along the floor of the carpal tunnel had specific imaging features. They were low signal on T1 and T2 with less enhancement postgadolinium than tophi seen in other locations. Computed tomography can also be very helpful (59) and frequently demonstrates characteristic stippled calcifications within these tophi. Patients with symptomatic median nerve compression from tophaceous gouty deposits are usually treated surgically with transverse ligament release and often debulking of the tophi (60, 61).
Autoimmune arthropathies such as rheumatoid arthritis may also cause carpal tunnel syndrome. Prevalence of carpal tunnel syndrome is estimated between 6% and 12% (62, 63). Tenosynovitis is often prominent in these cases. It is often not limited to the flexor compartment (Fig. 16.8). Characteristic arthropathy in the carpus is usually present. In general, open release is preferred to endoscopic treatment in active disease. In the large majority of cases, changes of tenosynovitis and tendinosis seen in the carpal tunnel are secondary to simple mechanical irritation. However, certain constellations of findings should alert the radiologist to the possibility of inflammatory etiology of the tenosynovitis. Increased severity of the tenosynovitis is frequently noted. This is visualized on MRI as marked synovial hypertrophy of the tendon sheath, often with peritendinous fluid and marked synovial enhancement. Strong evidence of an inflammatory etiology also includes tendon changes in the extensor compartments and focal osseous erosions.
Alterations in Fluid Balance
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree