Chapter 18 The elementary structure of the atom
Chapter contents
18.1 Aim
The aim of this chapter is to introduce the reader to the key components of an atom. The principal particles which form the nucleus will be identified, as will the factors which determine whether or not the nucleus is stable. The differing electron orbitals and the influence of the electron orbitals on the chemical properties of the material will be discussed. The consequences of transitions of electrons between orbitals will also be identified.
18.2 Introduction
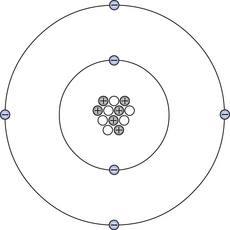
The planetary model of the atom was first described by Rutherford in 1911. It describes an atom consisting of a small, positively charged central nucleus (containing protons and neutrons) around which negatively charged electrons move in defined orbitals. This model can be used to illustrate the carbon atom, as shown in Figure 18.1. As we can see from the diagram, the nucleus of this atom consists of 12 elementary particles – six protons and six neutrons. These particles are bound together in a small volume of extremely high density – about three thousand million, million times greater than the density of water. The protons in the nucleus carry a positive charge and the electrons carry an equal negative charge, so this atom is electrically neutral – the neutrons carry no charge. The electrons are arranged in orbitals or shells called K, L, M … starting from the orbital closest to the nucleus. The K-shell can only contain two electrons and, in the case of the carbon atom, the L-shell contains the remaining four. Different atoms contain different numbers of protons and neutrons in the nucleus and different numbers of electron configurations; this will be discussed later in this chapter. The particles which make up the atom are very tiny and the atom consists largely of empty space. For example, if an atom were to be enlarged until it was the size of a house, the size of the nucleus would be about the size of a pin-head, although it contains 99.95% of the total mass of the atom.
The masses and charges of the subatomic particles which will be considered in this and later chapters are summarized in Table 18.1.
Table 18.1 Masses and charges of the main subatomic particles
| REST MASS | REST ENERGY | |||||
|---|---|---|---|---|---|---|
| PARTICLE | SYMBOL | kg | AMUa | MeV | CHARGEb | COMMENTS |
| Proton | p | 1.672×10−27 | 1.007 | 938 | +1 | Nucleon, i.e. present in the atomic nucleus |
| Neutron | n | 1.675×10−27 | 1.009 | 939 | 0 | Nucleon, i.e. present in the atomic nucleus |
| Alpha-particle | α | 6.645×10−27 | 4.003 | 3718 | +2 | Two protons and two neutrons |
| Ejected in α decay | ||||||
| Electron | e− or β− | 9.109×10−31 | 0.00055 or 1/1820 | 0.511 | −1 | Form stable discrete orbits around nuclei |
| Ejected from nucleus in β decay | ||||||
| Positron | e+ or β+ | 9.109×10−31 | 0.00055 or 1/1820 | 0.511 | +1 | Antiparticle of the electron – produces annihilation radiation when both meet |
| Pi meson | π+ | 2.480×10−28 | 0.150 | 139 | +1 | Keep the nucleus together (π0 and π− also exist) |
| Neutrino | ν | 0 | 0 | 0 | 0 | Emitted during β decay and electron capture |
| Very weak attenuation by matter | ||||||
| Photon or quantum | hv | – | – | – | 0 | Travels at 3×108 m.s−1 Forms part of the electromagnetic spectrum |
a 1 amu is 1 atomic mass unit which is one-twelfth of the mass of a neutral C126 atom.
b A charge of +1 is +1.602×10−19 coulomb.
18.3 The atomic nucleus
The number of protons and neutrons in the atomic nucleus determines both the mass and the charge of the nucleus and the configuration of electron orbitals of the atom. There are several important terms, which we will use in this and in following chapters of this text, that require definition at this stage. These terms, which will help us to understand atomic structure, are defined in Table 18.2.
Table 18.2 Terms used to describe a nucleus
| TERM | SYMBOL | DEFINITION |
|---|---|---|
| Nucleon | A proton or neutron within a nucleus | |
| Atomic number | Z | The number of protons in the nucleus |
| Atomic mass number | A | The total number of nucleons in the nucleus |
| Neutron number | N | The number of neutrons within the nucleus |
| Nuclide | A nucleus with a specific value of Z and A | |
| Element | E | A nucleus with a given value of Z |
| Isotope (of an element) | Any nucleus which contains the same number of protons as the given nucleus but has a different mass number | |
| Isobar | Any nucleus which has the same atomic mass number as another nucleus (i.e. has the same value of A) | |
| Radionuclide or radioisotope | Any nuclide or isotope which is radioactive |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree