Introduction
Looking back on the previous 30 years of progress in medical three-dimensional (3D) printing, it is astounding to consider just how far the field has come, with radiology playing a key, central role. Despite the dual infancies of medical imaging and 3D printing technologies in the late 1980s and early 1990s, a few pioneering individuals and organizations endeavored to bring the distinct technologies and communities together, integrating diverse expertise along with new hardware, software, and materials. From anatomic models for medical training and surgical planning, guides to directly assist with surgery, to permanent patient-matched implants, these increasingly deployed applications, in combination with new reimbursement strategies, regulatory involvement and support, as well as new workforce development and education programs in medicine, engineering, and technical support, have all grown from the initial seeds planted 30 years ago ( Fig. 16.1 ).
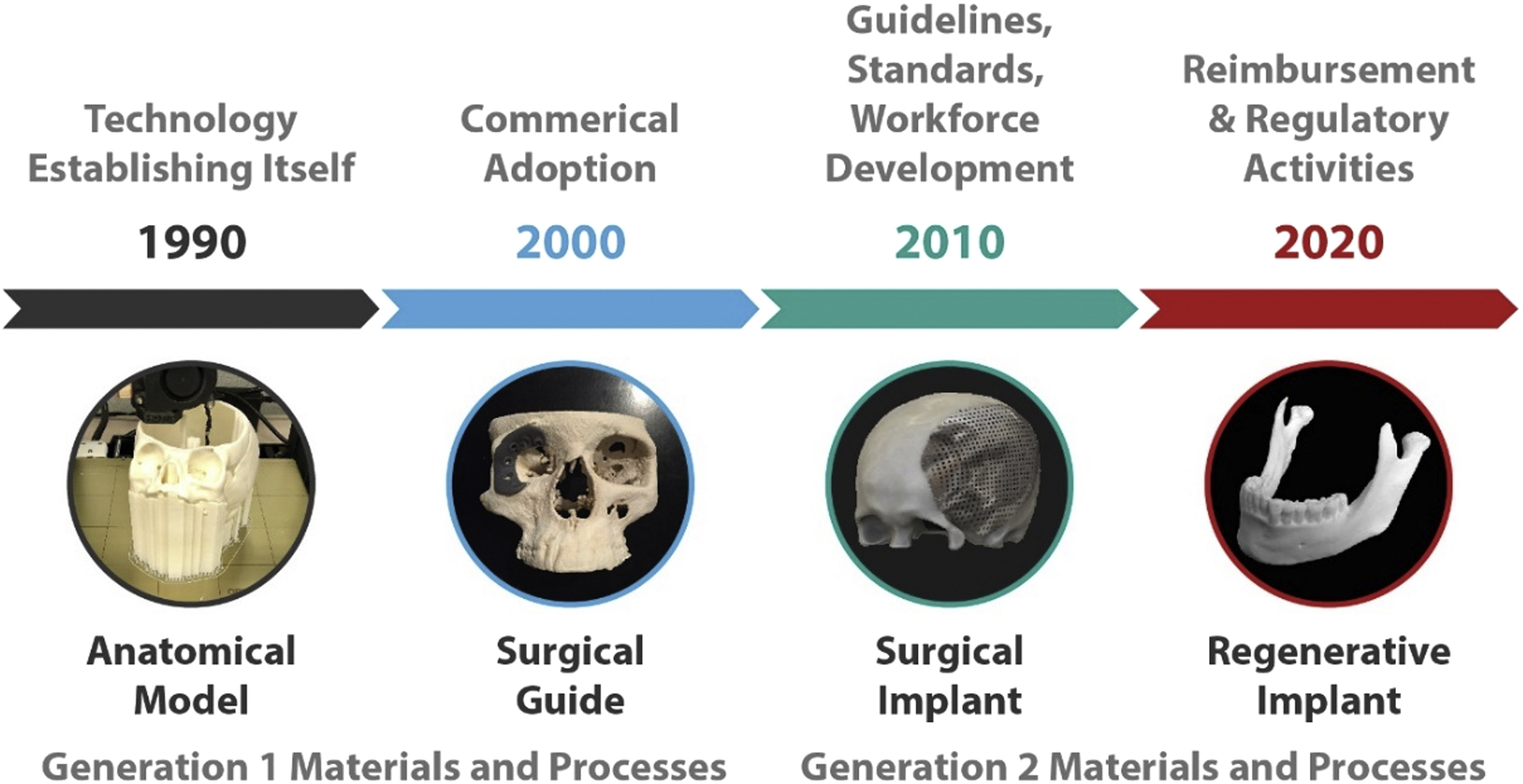
What about the next 30 years? With so much progress taking place in just the past few decades, let alone the past 5 years, it is no small feat to accurately speculate on what the next 30 years have in store. Using history as a guide, reasonable predictions can be made regarding the technological, clinical, and workforce trajectories of medical 3D printing in radiology. This chapter focuses on what the next 30 years of medical 3D printing might look like and which roles radiology and complimentary disciplines might play. Through the continued adoption and standardization of 3D printing for anatomic models and surgical guides to permanent implants, bioregenerative materials, and even living bioprinted tissues and organs, we can expect a medical transformation to take place over the next three decades, with radiology at its center. Given the rapid technological advancement and rates of adoption we have witnessed in just the past decade, it is reasonable to divide this discussion into several segments: near term (next 5 years), mid-near term (5–10 years), midterm (10–20 years), and long term (20–30 years).
Emerging Medical 3D Printing Technologies
Prior to delving into the future of medical 3D printing and radiology, it is beneficial to establish a foundational understanding of the emerging medical 3D printing technologies and create a reference system for which to categorize and discuss those technologies and their applications. Generally, medical 3D printing can be divided into five primary groups ( Fig. 16.2 ): educational anatomic models and surgical guides; static, permanent implants; acellular, regenerative biomaterials; cell-containing, living biological/tissue structures for tissue and organ repair, regeneration, and replacement, commonly referred to as “bioprinting”; and synthetic–biological, biomachine interface devices. Numerous intermediate classifications across these groups exist, including coating or modifying static implants with bioactive components; and adding living cells to originally acellular, regenerative biomaterials. It is important to distinguish the various categories of medical 3D printing because they vary with respect to (1) the types of hardware, software, and materials utilized; specific education and user expertise required; (2) the necessary manufacturing conditions and environment; (3) the existing or still developing standards; regulatory and reimbursement pathways; and (4) their means of use and intended application. It should be noted that acellular regenerative biomaterial and bioprinting are themselves emerging technologies and have not yet been clinically established.
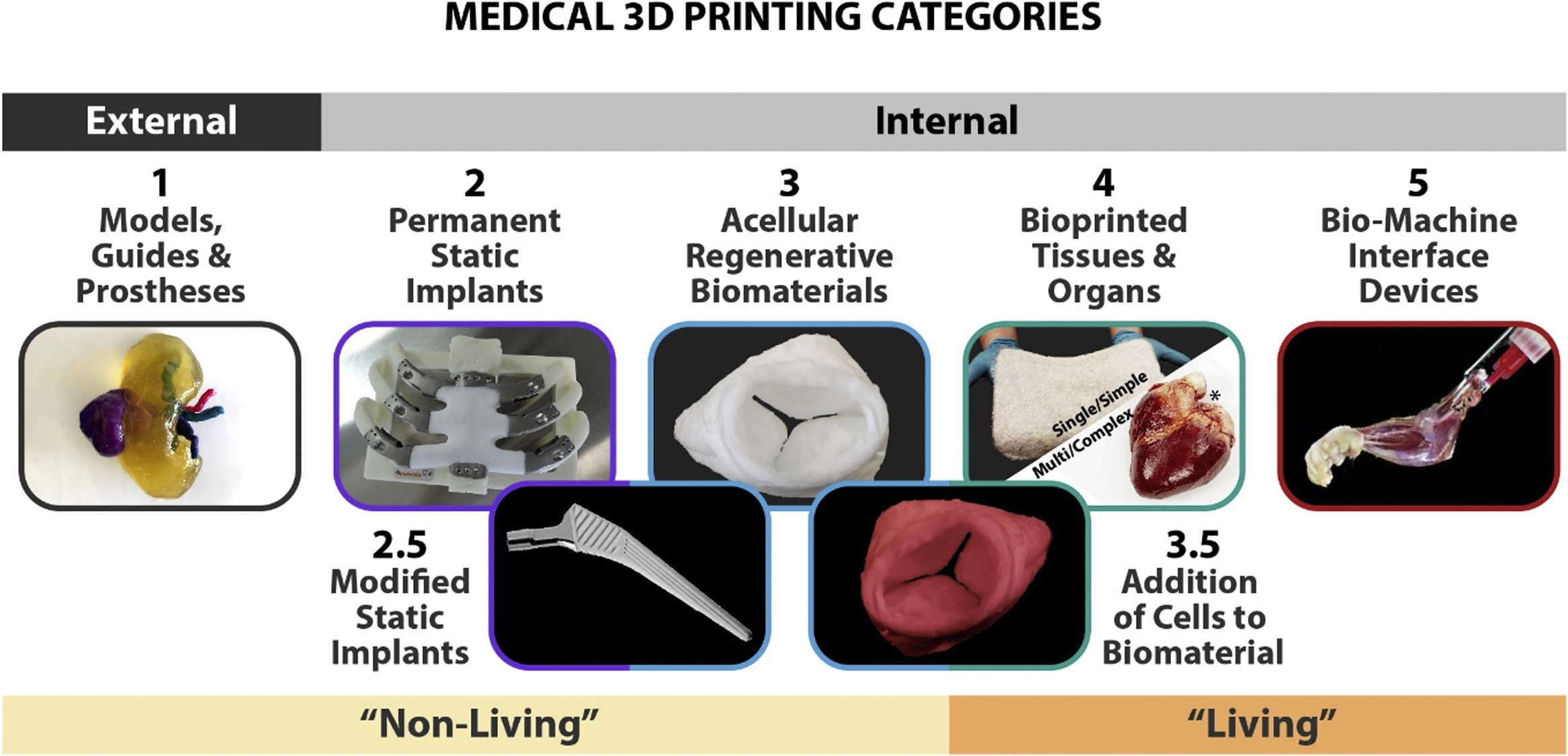
With a longer history than the other medical 3D printing categories, combined with the fact that they are not restricted by criteria and requirements associated with implantation, 3D printed anatomic models and surgical guides are presently the most widely utilized of the five categories. This is demonstrated, in part, by recent activities related to establishing reimbursement pathways for their use. In July 2019, Category III Current Procedural Terminology codes for 3D printed anatomic models and guides were released by the American Medical Association (see Chapter 8 ); and the joint Radiological Society of North America (RSNA)–American College of Radiology (ACR) 3D Printing Registry was created to track the use of clinical 3D printing. This registry collects anonymized 3D printing case information; clinical indications; model types including which anatomical parts, segmentation, and computer-aided design processing tools, and printing technologies utilized; and intended uses for the models. Information from this registry will be used to determine the Appropriateness Criteria for 3D printing as another modality of visualization. This reimbursement pathway currently only applies to select subcategories of medical 3D printing objects but will ultimately need to be extended to include all five categories of 3D printing as well as possibly integration with augmented and virtual reality technologies.
Current static, permanent implants are primarily employed for bone and hard tissue replacement, providing structural support rather than specific biological activity or function. This encompasses the 3D printed counterparts to many traditionally manufactured implant products made from established, relatively bioinert, yet biocompatible rigid plastics such as polyetheretherketone (PEEK), polyetherketoneketone (PEKK), and polymethylmethacrylate (PMMA) as well as metal alloys such as those based on titanium, stainless steel, or cobalt. Objects in this category are primarily produced via 3D printing methods that rely on heat, such as selective laser sintering/melting (SLS/SLM), electron beam melting (EBM), and more recently material extrusion techniques including fused deposition modeling (FDM) or fused filament fabrication (FFF), and direct ink writing (DIW). Although not yet commonly produced in the hospital, there is an increasing abundance of 3D printed static implantable devices that are regulatory cleared. Beyond the standard stock keeping units (SKUs), metal and rigid plastic parts are increasingly being 3D printed into patient-matched implants to treat complex, large-scale tissue resections of the pelvis, spine, rib cage, and skull. Importantly, these implants, although designed to integrate with surrounding tissues, are intended to remain relatively inert and unchanged (static) in the body for years, if not decades after implantation. For the sake of complete discussion, the bioactivity and integration potential of static permanent implants can be enhanced by coating or infusing them with more bioactive/biomimetic materials, such as calcium phosphates.
Acellular, regenerative biomaterial medical 3D printing is arguably the least widely discussed or understood of the first four categories. This lack of awareness derives from the understanding of existing medical materials and their primary purpose within the body—to physically and mechanically support the bioactive components, living cells, and tissues. However, emerging materials can play a strong bioactive role, beyond physical or mechanical support, even without requiring added cells. These objects are usually 3D printed via material extrusion processes, such as FDM and DIW. The biomaterials can be based on synthetically derived polymers, ceramics, and composites; naturally derived materials, such as collagens, glycosaminoglycans (GAGs), decellularized tissue extracellular matrices (ECM); or mixtures thereof. Several examples include noncell-encapsulating hydrogels, as well as the 3D painted (a variation of DIW) materials such as Hyperelastic Bone (bioceramic based) for hard-tissue repair and regeneration, , Fluffy-X (Advanced polymer based) for soft-tissue repair and regeneration, , Tissue Papers for targeted tissue repair and regeneration (Organ ECM based), 3D-Graphene for electrically reliant tissue repair (synthetic based), and mixtures thereof. , These materials not only have unique compositions but also unique nano/microstructures that, in combination, interact with surrounding tissues and cells to induce new target tissue formation while the implant itself degrades and transforms into natural, healthy tissue. Thus, acellular regenerative biomaterials, unlike static implants, are dynamic in nature and are designed to change and transform after implantation. Although not necessarily required, there is no technical barrier preventing the addition of cells to regenerative biomaterial objects prior to implantation , —an example of which would be a 3D printed meniscal structure seeded with patient-matched chondrocytes or stem cells immediately prior to implantation; or a gelatin-based 3D printed hydrogel seeded with ovarian-derived follicles (containing oocytes) prior to implantation that restore hormonal and reproductive function.
Cellular medical 3D printing, frequently referred to as “bioprinting” specifically involves 3D printing with live cells or tissue fragments to create structures that are intended to be implanted as is or further conditioned and matured prior to implantation. Being composed of acellular regenerative biomaterials, bioprinted objects are intended to repair and regenerate damaged or missing tissues and, potentially, partial or full organs. The materials in this case, unlike resins, powders, filaments, or particle suspensions, are living cells suspended in a cyto- and biocompatible medium, most often a hydrogel. Like acellular biomaterials, bioprinting processes are primarily material extrusion based (variations of DIW and material jetting), although new cytocompatible vat polymerization materials and processes are emerging. Although hydrogels based on synthetic and/or nonmammalian natural polymers such as polyethylene glycol, alginate, chitosan, cellulose, and others are common in academic research due to their low cost and relative ease of 3D printing, they, unless heavily modified, are not suitable for implantation due to the negative immunological responses they elicit. Mammalian-based hydrogels, such as those comprised of collagen, gelatin (denatured collagen), or decellularized tissue ECM, although more expensive and challenging to 3D print than their nonmammalian counterparts, are more suitable for implantation purposes and are presently the subject of broader, translational biofabrication efforts.
Regardless of the material medium or process used, the comprising cell viability and health is of utmost importance, and can be negatively impacted by a variety of environmental (temperature, pressure, humidity), processing (shear stress from extrusion), and post 3D printing handling, transport, and storage—all of which must be taken into account when 3D printing living cells. Additionally, because these structures contain living cells and lack an immune system, they cannot be terminally sterilized or fend off pathogenic contaminants. Thus, bioprinting living structures for implantation should be done using sterile raw materials, within a sterile environment, using sterile processes. Regarding cell sourcing, there are significant ongoing efforts in academia, government, and industry research labs to establish their sourcing, quality, consistency, and bioactivity whether they be autologous patient primary cells, adult stem cells, induced pluripotent stem cells, or induced pluripotent-derived cells.
Biomachine interface medical 3D printing broadly encompasses technologies and applications that are beyond 3D printing static devices or living tissues and organs. This includes 3D printing of integrated biological-machine structures as well as advanced engineered and augmented tissues and organs. Although presently a collection of ideas with minimal completed proofs of concept, biomachine interface medical 3D printing is a logical technological progression of multitissue 3D printing combined with nonmedical 3D printing technologies, including advanced electronics, energy generation, and storage devices. Generally, this category represents the intersection of medical with industrial manufacturing, and although it will still be at least 30 years until even a solid proof of concept is realized, not to mention many more years until broad adoption, this is an area of which current, younger radiologists and medical professionals should be aware.
Despite the stark contrasts among these five categories, they share numerous similarities in the broader context of in-hospital 3D printing. Regardless of application, each of the five approaches requires (or will require) precise and accurate input data, usually derived from the acquisition, interpretation, and segmentation of patient imaging studies, as discussed in previous chapters. Whether that imaging data are derived from CT, MRI, ultrasound, surface scanning, nuclear medicine, other imaging modalities, or combinations thereof, they are the basis for the digital inputs for models, guides, permanent implants, and even future tissue regenerative, bioprinted, and biomachine interface structures. The forms that the digital inputs take may vary substantially between objects created for surgical planning versus bioprinted objects containing a variety of living cells and tissue types. For example, the primary characteristic of surgical models and guides is to emulate the form and architecture of the target tissue/organ based on imaging data. This will hold true for bioprinted structures as well, where the form and architecture will need to be emulated to match the patient. However, in addition to recreating the form factor, possibly down to the vascular level, bioprinted structures will also have to recreate the target tissue/organ’s composition and properties. This will not only require digital inputs derived from existing imaging technologies but will also require additional complementary and spatially correlated compositional digital inputs. Thus, the clinical implementation of bioprinting technologies is reliant on the parallel development of imaging technologies that can be used to ascertain biological composition and properties.
It is also important to mention the distinct expertise and knowledge required to pursue each of the five categories. Progressing from models and guides to bioprinted and biomachine structures, there is a gradual transition from pure visualization and image processing to mechanical engineering, materials engineering, tissue engineering, and electrical/systems engineering—all of which are currently distinct disciplines, but share overlaps that may present opportunities for new workforce development and engineering fields in the future.
The Case for In-Hospital 3D Printing
As of 2020, many hospitals and healthcare institutions throughout the United States and across the world utilize medical 3D printing to create anatomic models and surgical guides in-house for a variety of clinical applications—this is increasingly becoming the norm rather than the exception. However, for organizations that rarely utilize 3D printing for these applications and do not have the required internal equipment or expertise, it is logical to use these third parties, as they have the hardware, software, and know-how (in combination with clinician input). Despite this, there are good reasons why anatomic model and surgical guide 3D printing in the hospital have become much more common. These reasons have been discussed at length in previous chapters but did not take into consideration the added layers of complexity of bringing in-house the other categories of medical 3D printing such as implant 3D printing. Given the differing and added hardware, software, and materials requirements as well as the varying technical know-how, increased risk, and currently uncertain regulatory complexities surrounding in-hospital–fabricated implants, why would anyone want to go through the effort to produce them at the point-of-care rather than relying on external, third-party entities to do so? The answer comes down to four factors: time/cost, availability, logistical practicality, and patient care.
Certainly, the most immediate benefit of producing 3D printed anatomic models and guides onsite is time. If patient data can go from digital imaging data, to physical creation, to use within a day or two, rather than waiting on third-party schedules, manufacturing queues, and shipping, then there is a clear case to be made for efficiency and obvious advantages to the patient. These time benefits are further compounded in the case of 3D printed static, permanent, and acellular regenerative biomaterial implants, where off-the-shelf SKUs simply are not available or do not exist anywhere, and the lead time for receiving a patient-matched implantable device from a third party may be several days to weeks. For patients with critical needs, this period might be the difference between life and death, and for the hospital, will result in added weeks of medical care and increased operating costs. However, the time-saving advantages to producing patient-matched static and acellular regenerative implants in the hospital do not necessarily extend to bioprinted objects, which require large numbers of cells and possible conditioning and maturation times. Acquiring and expanding cells to the sufficient number to 3D print an object that might be required for critical care could take weeks, and conditioning and maturing that structure after printing might take several weeks more. Additionally, since bioprinted objects are living and there are currently no established methods for storing or transporting living tissues without function loss, their postproduction lifetime, like transplanted tissues and organs, is currently limited.
Implant availability, or lack thereof, is another factor that will drive the adoption of in-hospital implant medical 3D printing. As of 2020, the majority of implantable products, regardless of their indicated use or means of production, are mass-produced, and even available in a variety of discrete-sized SKUs. These mass-produced devices and their specific sizes are manufactured in part because there is a large enough demand and patient population to justify their development from a business perspective. However, standard-sized implant products are not appropriate for all patient populations or individual patients. In these instances, three options exist: use whichever implant is available and is the closest fit despite its inappropriateness for the patient, forego the implant and find some other way to treat the patient, or design and manufacture a patient-matched implant. As discussed above, although third-party static implant manufacturing is becoming increasingly available, this still needs to be balanced with time and patient need. For example, a noncritical mandibular implant may be needed for an upcoming surgical procedure—in this case, the time required for a third party to manufacture and ship the implant may be acceptable. In another case, a critical need, specifically designed bronchiotracheal implant may be needed to treat a neonatal infant who may otherwise not survive a week. This latter scenario is an example of compassionate use and is perhaps emblematic of the real value of in-hospital implant medical 3D printing. This logic can be extended to acellular regenerative biomaterials 3D printing and bioprinting as well, although at the moment, these types of implants are not necessarily commercially available from third parties. Regarding bioprinting, it will be unlikely that large supplies of commercially manufactured tissues and organs, compatible with all patient populations, will be readily available for purchase. Thus, like the bronchiotracheal example given previously, they will likely need to be made as needed—and time will play a major factor for their use.
Logistically, it is impractical for a hospital, regardless of its economic status and administrative capabilities, to store and manage an extensive inventory of implantable products of varying SKUs, many of which may never end up being used. This is one of the major factors that will drive adoption of static implant medical 3D printing in the hospital—reduce standing inventory and waste. Although this argument is also possibly applicable to acellular regenerative and bioprinted implants, the logistical concerns surrounding them are more extensive. Currently, shipping live tissues and organs for transplantation is no simple task; it must be done rapidly, with great care, and is still ultimately limited by distance and time. These same challenges will exist for bioprinted tissues and organs as well, with the added complication that, in all likelihood, those tissues and organs will require specific cells from the patient as manufacturing raw material, therefore requiring an additional shipping and handling step. As alluded to previously, until tissue and organ transport and preservation challenges are effectively resolved, such structures will need to be produced near the target patient.
Finally, if it was not believed that patient care could be improved through technological medical advances, then there would ultimately be no major case for in-hospital manufacturing of implants. While obtaining the patient-matched implants from third-party manufacturers will continue to remain a significant part of personalized healthcare, there will remain many instances where patient care can be substantially improved through in-hospital implant manufacturing—and in these instances, radiologists will be the primary facilitator.
A Word on Sterilization and Quality Manufacturing at the Hospital
3D printed medical implants, whether they are composed of alloys, plastics, regenerative biomaterials, and/or living cells, must be free of pathogens prior to use and must be manufactured to the necessary level of verifiable and biocompatible quality. Quality and sterilization of static implants have been discussed at length in previous chapters, but it is worth reiterating the importance of ensuring sterility of any 3D printed object that is intended to come into contact with the patient or enter a sterile surgical field. Protocols and standards are currently being developed for sterilization of in-hospital–produced static implant devices, which do not substantially deviate from existing chemical or thermal sterilization methods, but material compatibility must be taken into account before introducing the object to the sterilization procedure. It must also be known whether or not the sterilization process affects the dimension or mechanical properties of the object, as such changes can have downstream adverse influence on the performance of surgical guides and anatomic models.
The characteristic stability that enables static implants to remain relatively unchanged in the body after implantation also permits them to be sterilized using already established methods, such as dry or hot heat (autoclaving), gamma irradiation, ethylene oxide (EtO), hydrogen peroxide (H 2 O 2 ), and others. These processes have an established history, and most hospitals and clinics have some sterilization capabilities. However, these established methods are not readily compatible with acellular regenerative biomaterials. For example, excessive heat or free radical chemical sterilization such as EtO and H 2 O 2 will alter if not destroy the base materials. This incompatibility with emerging advanced biomaterials can be addressed through the adoption of novel sterilization techniques, such as peracetic acid liquid/vapor exposure and supercritical CO 2 . Fortunately, these emerging processes and hardware are scalable and could be incorporated into hospital environments in a manner analogous to current EtO and autoclaving equipment and processing—allowing for onsite sterilization of in-hospital 3D printed acellular, regenerative implants. However, as of 2020, the FDA still considers these emerging techniques as novel, and they have only been used in conjunction with a few cleared products. Fortunately, the FDA also recognizes the increasing need to reduce reliance on methods such as EtO, and in 2019 launched an Innovation in Sterilization Challenge to work with industry partners to explore and establish novel sterilization techniques for broader use. Ideally, these efforts will establish new approaches to sterilization that are not only compatible with acellular regenerative biomaterials but can also be readily incorporated into existing hospital sterilization infrastructure.
Sterility is further complicated with respect to bioprinted structures, which by their very nature contain microorganisms (human cells) that need to remain viable and functional for the implant to survive and serve its purpose. Thus, sterilization, using traditional or emerging novel methods, is fundamentally not compatible with bioprinted structures. Bioprinted structures intended for implantation, regardless of their complexity, must be manufactured and handled in a fully aseptic and sterile environment and manner. Additionally, raw materials and components used in the bioprinting process, including the cells, media, water, gel materials, plasticware, glassware, metal ware, etc., must all be sterile prior to use. These sterility requirements impart substantial manufacturing, infrastructure, expertise, and practically burden on in-hospital bioprinting, and are the primary reasons why this form of medical 3D printing will not be established within individual hospitals in the near future.
The Next 5 Years
In the next 5 years, we can expect to see the continuation of current trends related to medical 3D printing of anatomic models and surgical guides as well as the initial adoption of 3D printing to create static permanent implants ( Fig. 16.3 ). Unlike previous years, where adoption was primarily driven and supported by independent physician activities, hospitals and healthcare institutions will likely begin to provide additional internal support for these activities. This increasing support will be driven by four factors: (1) Hospitals and other healthcare centers are beginning to recognize net positive effect on the long-term bottom-line of medical 3D printing, despite the upfront costs; (2) in an effort to remain competitive with state-of-the-art capabilities, hospitals and medical centers will begin using 3D printing as a means of differentiation and marketing leverage; (3) there are an increasing number of doctors, nurses, engineers, and technicians being exposed to medical 3D printing during their education and training who are entering the healthcare workforce; and (4) related reimbursement pathways for 3D printed surgical models and guides will be increasingly established. Additionally, while the relative costs for SLA, FDM, and certain material jetting 3D printing hardware and materials are decreasing, their ease of use and accessibility are increasing, reducing the initial technical barrier required to initiate operations.