5 The Gastrointestinal Tract For many decades, imaging of the gastrointestinal (GI) tract was the domain of radiographic procedures, notably double-contrast techniques. Today, with endoscopy having evolved into a serious competitor, even the small intestine is no longer the undisputed territory of radiographic techniques. New perspectives are also opened up by the advances made in CT, which offers good visualization of the bowel wall and provides more diagnostic information than conventional X-ray examinations and endoscopy because it also allows direct evaluation of the tissues outside the GI tract. MRI, the other major cross-sectional imaging modality, was of little use for GI imaging in its early years because of long acquisition times. The situation has changed with the development of fast MR pulse sequences and parallel imaging techniques. Nevertheless, CT is still preferred to MRI for most diagnostic applications in the GI tract because it is superior in terms of speed, resolution, multiplanar image reformation, imaging of the entire abdomen in a single scan, and absence of artifacts. On the other hand, MRI has the crucial advantage of not involving ionizing radiation and is therefore generally preferred in young persons and pregnant women, for follow-up examinations, and in dynamic studies. With its high intrinsic contrast, MRI has great potential for evaluating inflammatory and neoplastic processes of the bowel. MR enteroclysis1–6 has been used since 1997 to evaluate and follow up complications of Crohn disease that require surgery. MR colonography (MRC) is still under investigation for colorectal screening7–12 (Table 5.1). MRI is also a promising modality for diagnosing appendicitis in cases where ultrasound findings are inconclusive and imaging does not delay surgery. Finally, MRI enables functional assessment in esophageal reflux, gastric emptying disorders, adhesions, pelvic floor hernias, and defecation disorders. Fasting for 12 h is sufficient before MRI of the stomach or small intestine, but active bowel preparation is needed for imaging of the colon. Bowel cleansing for MR colonography is the same as for colonoscopy: using, for example, a polyethylene glycol electrolyte solution (PEG-ES; Golytely, Klean-Prep).13 For adequate cleansing, patients should start drinking the solution at 3 p.m. on the day before the examination and restrict themselves to clear liquids. The question of whether less strict bowel preparation or dietary measures alone may be sufficient before intestinal MRI7 needs to be investigated in further studies. An advantage of MRI over unenhanced CT is that evaluation of the intestinal wall is not degraded by residues of the cleansing solution.
W. Luboldt and M. Laniado
Introduction
Indications
Imaging Technique
Patient Preparation
| Indication (examination) | Sequence (planes) | Comment |
| Crohn disease (MR enteroclysis) | HASTE, TrueFISP (?), contrast-enhanced VIBE (abdomen: coronal; pelvis: axial) | To evaluate complications (see Table 5.3) |
| Colorectal screening (MR colonography) | HASTE, TrueFISP (?), contrast-enhanced VIBE (abdomen: coronal; pelvis: axial) | Under investigation |
| Appendicitis | HASTE, HASTE-IRM, contrast-enhanced VIBE (?) (identification of the appendix with axial and coronal | If clinical findings are unclear and MRI does not delay surgery |
| HASTE and HASTE-IRM; if still inconclusive, use additional focused contrast-enhanced axial VIBE for MPR) |
Start VIBE(volume-interpolated breath-hold examination) sequence ca. 40 s after IV administration of spasmolytic and contrast medium and repeat sequence 2–4 times to have alternative images for interpretation in case of peristalsis artifacts. If peristalsis artifacts are encountered, reduce scan time by decreasing number of slices and increasing slice thickness.
Oral/Rectal Contrast Media
Evaluation of the GI tract is possible only after adequate distention (see Fig. 5.2). Although water alone is sufficient for distention and delineation of the gastric or intestinal wall, 2.5 % mannitol needs to be added to prevent premature osmotic absorption of the water when the small bowel is examined. Alternatively, small bowel loops can be distended and delineated with PEG-ES, as used for bowel lavage before colonoscopy.6 When PEG-ES is used, the MRI scans must be timed such that enough of the solution is retained in the small bowel without inducing a desire to defecate. If the contrast solution is administered through a nasojejunal tube (MR enteroclysis), filling of the small bowel can be evaluated in a dynamic MR study.5 Contrast administration through a nasal tube is more efficient, but oral intake is also possible, since the tube cannot be positioned under MRI guidance and some patients refuse intubation.
For MR colonography, a rectal water enema administered with a bag suspended 1 m above the patient is theoretically superior to air insufflation because water results in better delineation of the bowel wall on HASTE sequences than air (Fig. 5.1) and possibly also better distention (Fig. 5.2). However, as with CT colonography, air insufflation is more convenient and is preferred as long as there is no clear evidence of its disadvantages.
Spasmolysis
Unless butylscopolamine is contraindicated, intravenous spasmolysis with 20 mg of Buscopan is recommended to maximize bowel distention and minimize peristalsis-related artifacts. The maximum effect of butylscopolamine occurs as early as 1 min after intravenous injection and persists for only a few minutes. Butylscopolamine should therefore be saved for bolus administration immediately before the scan for which effective suppression of peristalsis is most important. Imaging should then start 1 min after bolus injection and should be completed in less than 10 min.
If butylscopolamine is not approved in a country or is contraindicated in a patient (prostatic hyperplasia, narrow angle glaucoma, tachyarrhythmia, myasthenia gravis), an unenhanced VIBE sequence can be acquired to estimate the extent of peristaltic artifacts. If reduction of persistaltic artifacts is deemed necessary, the patient should be given 1 mg of glucagon.
Pulse Sequences
Abdomen
As abdominal imaging should be performed with breath-holding, there are basically three sequences that can be used: HASTE, TrueFISP, and 3D GRE (VIBE) (see Fig. 5.1). The HASTE sequence can also be obtained with the patient breathing freely and is therefore well suited for orientation and the initial search for pathology. A TrueFISP sequence allows evaluation of vessel patency and differentiation of vessels and lymph nodes. Moreover, it can serve to identify infiltration of adjacent organs by exploiting chemical shift effects that occur at interfaces between soft tissue and fat. Presence of this effect indicates an intact fat plane between two adjacent organs. A VIBE sequence should be started ca. 1 min after butylscopolamine administration and 40 s after intravenous contrast injection (nonspecific Gd-based contrast medium at a dose of 0.1 mmol Gd per kg body weight; e.g., Magnevist or Dotarem). Recommended practice is to repeat the sequence immediately to have a second data set in case the first one is degraded by motion artifacts. As with CT, the 3D data set can be used for multiplanar reconstruction. In the abdomen, images should be acquired in the coronal plane for three reasons:
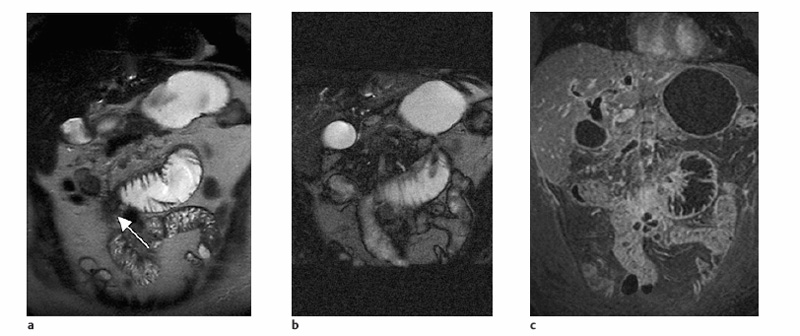
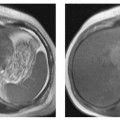
Fig. 5.2a, b Pitfall. Coronal T2w HASTE images obtained with inadequate rectal air insufflation (a) and after adequate air insufflation (b). With poor colonic distention (a), a nondistended segment mimics a tumor, which is shown to be a fold on the image obtained after adequate distention (b).
- It is the most suitable for following the intestine.
- Long, continuous segments of the bowel wall can be evaluated.
- The regular pattern of opposing haustra in the coronal plane facilitates identification of polyps.
Pelvis
Artifacts due to respiratory motion are usually less severe in the pelvis, and breath-hold imaging is not necessary in most instances. The pelvis can therefore be imaged using a TSE or SE sequence with scan times longer than 25 s. STIR imaging is the method of choice for evaluating fistulas. With the improved coils available today, most diagnostic questions can be answered using a fast T2w sequence, which markedly reduces scan time and does not require contrast administration (see Fig. 5.1). The rectum and sigmoid colon are best evaluated on axial images.
Imaging Strategy
Gastrointestinal imaging is performed with the patient in a comfortable supine position using a surface coil combined with the coil elements integrated in the table.
As with CT, an overview of the abdomen and pelvis should be obtained in the axial plane, for which a HASTE sequence is used. While coronal imaging is preferred for further evaluation of the abdominal portion of the GI tract, the pelvic part is primarily imaged in the axial plane. Double angulation along the terminal ileum is helpful in patients with colitis involving this bowel segment. Data sets acquired with a VIBE sequence allow multiplanar reconstruction images to be retrospectively generated in any desired plane. If peristaltic artifacts are present and it is not possible to reduce the scan volume, the number of slices can be reduced by increasing the slice thickness (1.5–2 mm).
Since there is not much motion in the pelvic region, spatial resolution can be improved by acquiring the VIBE sequence during shallow breathing. Higher resolution improves multiplanar reconstruction for image interpretation; the rectum can then be evaluated on sagittal reconstructions instead of performing primary sagittal imaging of this region. To maintain adequate SNR, the number of acquisitions should also be increased if the slice thickness is decreased (minimum of 1 mm) and the matrix increased.
When a VIBE sequence is used, intravenous contrast administration is needed to evaluate the bowel wall (see Fig. 5.1). Timing of postcontrast imaging is not very critical as signal enhancement of colorectal masses persists for a few minutes. However, there should be a delay of at least 40 s after the start of contrast administration to ensure that the contrast medium has reached the target site when the central k-space lines are sampled. It is good practice to repeat the VIBE sequence as a dynamic series to have backup images if others are degraded by artifacts.
MRI Appearance of Pathologic Entities
Stomach
Adenocarcinoma. Adenocarcinoma is the most common malignant tumor of the stomach. Nearly half of all gastric carcinomas occur in the region of the gastroesophageal junction. Three growth patterns are distinguished: localized, circular, and diffusely infiltrating (linitis plastica, “leather bottle” stomach). Thickening of the gastric wall may be minimal to very pronounced (>4 cm) and correlates with transmural tumor extension. A wall thickness > 2 cm is highly indicative of extragastric spread. Proximal cancer spreads along the gastrosplenic ligament to the left lobe of liver, the diaphragm, and the spleen, while distal cancer may invade the pancreas. Lymph node metastases occur in perigastric, celiac trunk, hepatoduodenal ligament, retropancreatic, mesenteric root, and para-aortic nodes. Advanced disease is characterized by tumor spread to the major omentum, peritoneum, and ovaries (Krukenberg tumor). TNM staging of gastric cancer is summarized in Table 5.2.
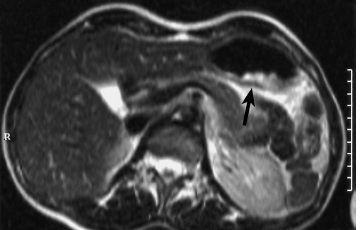
Fig. 5.3 Gastric carcinoma. The tumor has high SI (arrow) on T2w TSE image.
Gastric Lymphoma. The stomach is the most common site of lymphoma in the GI tract (especially in non-Hodgkin lymphoma), with gastric lymphoma accounting for 3–5% of malignant stomach tumors. While there may be isolated lymphoma of the stomach, concomitant manifestation in other parts of the GI tract is more common. Since lymphoma is primarily submucosal, it may be difficult to diagnose by endoscopy. Gastric lymphoma can directly infiltrate perigastric fat and adjacent organs, be associated with regional lymphadenopathy, and/or disseminate into the peritoneal cavity. Unlike adenocarcinoma, lymphoma often involves the entire stomach and may be confined to the wall, even if the wall is > 4 cm thick.
Leiomyosarcoma. This rare tumor accounts for 1–3 % of malignant gastric neoplasms. The primary tumor is usually large and round to ellipsoid in shape. In contrast to other gastric tumors, wall thickening may be absent because the bulk of the tumor is often outside the stomach. Leiomyosarcomas metastasize by contiguous extension to adjacent organs, peritoneal seeding, or hematogenous spread (liver, lungs, bone). Metastasis to perigastric and para-aortic lymph nodes is uncommon.
Gastric Metastasis. The most common primaries are malignant melanoma, breast cancer, and lung cancer. Gastric metastases from breast cancer occasionally manifest as diffuse wall thickening, which cannot be differentiated from primary adenocarcinoma. Squamous cell carcinoma of the distal esophagus involves the cardia in 15 % of cases. Cancer of the transverse colon and pancreas can directly invade the stomach wall along the gastrocolic and gastrosplenic ligament, respectively.
Leiomyoma. This is the most common benign tumor of the stomach and arises in the submucosa. It is usually asymptomatic. Leiomyomas > 5 cm frequently undergo ulceration and hemorrhage. They can then be diagnosed on T1w images by the presence of high-signal-intensity blood (between 2 days and 4 weeks) or susceptibility artifacts produced by iron-containing blood products such as hemosiderin. Other conditions that can cause thickening of the gastric wall are Ménétrier disease (typically of the fundus and corpus) and Crohn disease (typically of the antrum).
MRI
The patient is asked to drink as much water as possible 5 min before the examination and is given an intravenous injection of butylscopolamine to ensure maximum distention of the stomach. Most gastric cancers are hyperintense relative to the stomach wall on T2w images (Fig. 5.3). However, they may also be isointense, which is why a contrast-enhanced study is recommended in patients with suspected gastric cancer (Fig. 5.4). Chemical shift imaging appears well suited for local cancer staging (Fig. 5.5). The destructive interaction between fat and water signals on opposed-phase (OP) images, which are obtained at specific TEs (2.1 ms or an odd-numbered multiple at 1.5 T), produces a dark line at the interface between the gastric wall and surrounding fat. Interruption of this line on OP images is a fairly reliable indicator of tumor extension to adjacent organs (Fig. 5.5).
Small Intestine
Tumors of the small intestine are rare, accounting for ca. 6 % of gastrointestinal neoplasms. One-third are found in the duodenum, and most of the remaining two-thirds are located in the terminal ileum. The most common benign tumors of the small intestine are leiomyomas, adenomas, and lipomas. Malignant small-bowel tumors include adenocarcinoma, leiomyosarcoma, lymphoma, neurofibrosarcoma, carcinoids, gastrointestinal stromal tumors (GIST), and metastases (e.g., from the lungs, breasts, or malignant melanomas). Neoplasms of the small intestine may cause intussusception.
Leiomyoma and Leiosarcoma. These are highly vascular tumors, which often undergo central necrosis and commonly present with profuse bleeding. Since their growth is eccentric, they typically have an extraluminal component. Leiomyomas and leiosarcomas are often difficult to distinguish on the basis of their imaging appearance.
Adenocarcinoma and Lymphoma.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree