The Gastrointestinal Tract
Barbara Hall-Terracciano
|
OBJECTIVES
Understand the anatomy of the gastrointestinal tract, including the five sonographic layers of the bowel wall.
Define the process for sonographic evaluation of the gastrointestinal tract, including examination preparation and technique.
Know the transabdominal and endoluminal approaches include the endovaginal and the endorectal ultrasound approach as well as the choice of the transducer for the examination.
Describe the esophagus, stomach, small bowel, appendix, and colon.
Discuss disorders of the esophagus, stomach, small bowel, appendix, and colon that can be sonographically visualized.
KEY TERMS
adenocarcinoma
appendiceal abscess
appendicitis
carcinoid tumor
Crohn disease
diverticulosis
duodenal ulcer
gastric carcinoma
gastric dilatation
gastric ulcer disease
gastritis
gastrointestinal stromal tumor
hematoma
intussusception
leiomyoma
leiomyosarcoma
lymphoma
mucocele
peptic ulcer
small bowel edema
small bowel obstruction
squamous cell carcinoma
ulcerative colitis
volvulus
GLOSSARY
appendicolith fecolith or calcification found in the appendiceal lumen
atonic without muscular tone
Crohn disease inflammatory bowel disease causing chronic inflammation of the gastrointestinal tract
duodenal web complete or incomplete obstruction at the duodenum because of membranous web or intraluminal diverticulum
dysphagia difficulty swallowing
elastography technique for identifying changes in elasticity of soft tissue resulting from diseases processes
endoluminal within the lumen of a tubular organ such as the gastrointestinal tract
endoscope (sonoendoscope) flexible lighted tube with high-frequency sound waves that produce images of the digestive tract walls and lining in addition to adjacent structures such as lymph nodes, major blood vessels, pancreas, liver, and chest
endosonography sonography done using an ultrasound transducer attached to an endoscope
esophagogastroduodenoscopy (EGD) endoscopic procedure to depict the esophagus, stomach, and first portion of the duodenum
glucagon agent used to achieve bowel aperistalsis
graded compression technique where transducer is slowly and firmly compressed against the abdominal wall to displace inferior structures, generally bowel loops
gut digestive tract
H. pylori (Helicobacter pylori) bacteria that live in the digestive tract causing sores/ulcers, which may evolve into cancer
hematochezia discharge of fresh (bright red) blood through the anus
ileus failure of the intestine to propel its contents because of diminished motility
ligament of Treitz suspensory ligament at the level of the duodenal junction
McBurney point point over the right side of the abdomen that is one-third of the distance from the anterior superior iliac spine to the umbilicus
Ménétrier disease rare condition of the stomach where overgrowth of mucous cells in the membrane lining results in large gastric folds leading to decreased or absent acid production
midaxillary line vertical line from the midaxilla toward the iliac crest
optical endoscopy rigid or flexible tube with a fiberoptic light source designed to investigate the inside of bowel, organs, and lung structures
peristalsis rhythmic dilatation and contraction that propels the contents of the gastrointestinal tract
peritonitis inflammation of the covering of the peritoneum that covers and supports abdominal organs
polyp growth on a stalk protruding from mucous membranes
ulcer an erosion in the mucosal layer of the wall of the gastrointestinal tract; frequently located in the stomach or duodenum
volvulus abnormal twisting of the intestines that can lead to obstruction, gangrene, perforation, and peritonitis
Zollinger-Ellison syndrome rare condition of secreting tumors (gastrinomas) forming in the pancreas or duodenum causing abnormally increased stomach acid
The gastrointestinal (GI) tract, also known as the alimentary canal, includes the lips, mouth, pharynx, esophagus, stomach, small intestine, appendix, and colon through to the anus. Sonographic examination of the GI tract began in the 1980s using endoscopic ultrasonography (EUS) and the transabdominal approach. Forty years later, those methods, as well as the endorectal and endovaginal approaches, are still utilized. All methods have continued to gain diagnostic importance because of technical equipment advancements and increased sonographer GI exposure, which has led to indispensable experience and diagnostic accuracy. At its inception, GI ultrasound was not often performed by sonographers because of the difficulty in evaluating the GI structures by an inexperienced operator; however, continued sonographer exposure to GI studies has contributed to expanded use of this modality. Technical advances in ultrasound systems have included options such as harmonic imaging, beam steering, and speckle reduction in addition to high-resolution transducers, which also increase the visualization of GI structure and added diagnostic accuracy. GI sonography is extremely useful and appreciated in countries where access to expensive testing—such as endoscopy, computerized tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET)—is limited and in areas where contrast studies are commonly performed.
SONOGRAPHIC GASTROINTESTINAL EXAMINATION TECHNIQUE
Real-time transducers provide the sonographic equivalent of fluoroscopy. Peristaltic activity visible on real-time examinations is impossible to capture on still images but can be acquired with cine clips. Movement of the GI tract is invaluable to the sonographer who is evaluating its function and ruling out an anomaly. Peristalsis of the bowel is expected, and when viewed by an experienced sonographer, it can help with a diagnosis.
Although the GI tract is not usually the primary focus of an abdominal sonogram, clinicians value GI information when abnormalities are discovered that may be the cause of abdominal symptoms. Incidental information, such as excessive or no peristaltic activity, increased intraluminal fluid contents, suspected bowel dilatation, and inflammation, is critical to making an accurate diagnosis. A targeted GI ultrasound can provide information about the GI tract, in many cases, similar to a CT and MRI.
Three classes of sonographic exam performed on the GI tract are transabdominal, transvaginal/transrectal, and endoluminal sonography/endoscopic ultrasonography (EUS). Transabdominal sonography is the traditional method, often used as a screening procedure to evaluate abdominal or pelvic pain and cause of fever, rebound tenderness, and palpable abdominal mass by evaluating the bowel wall thickness (BWT) and the pattern of echogenicity, vascularity, and mobility. Other reasons for GI ultrasound include follow-up of a previous GI sonography exam; abnormal laboratory data; or the results from radiography, CT, MRI, and/or PET.
Endoluminal sonography is not widely used by general sonographers. It is performed by endosonographers and gastroenterologists using specialized endoscopes. The endoscope or linear endoscope is specially designed with a sonographic transducer at its tip, which produces sonographic images from within the lumen and directly adjacent
to the GI wall. An optical endoscope evaluates the lumen with a light. Endoluminal sonographic examinations are directed specifically at the bowel or surrounding structures. The coupling of sonography and endoscopy has allowed physicians to better visualize and stage cancers within or adjacent to the GI tract and to evaluate the depth of lesions within the intestinal wall. Transesophageal, transduodenal, and transgastric sonographic evaluations can detect abnormalities in the pancreatobiliary system, paraluminal and intramural lesions including lymphadenopathies, ascites, and lesions in the left liver lobe; and reasons for rectal bleeding, diarrhea, hematochezia, bowel obstruction, and change of bowel habit. Linear endoscopes have brought a new landscape to EUS because of their ability to track a needle in real time across the image plane into a target lesion. This is known as endoluminal ultrasound with fine needle aspiration (EUS-FNA). The electronic instruments also permit the use of Doppler technology to assess vascular flow.1 Endosonography has been performed primarily on the upper GI tract: the esophagus, stomach, and duodenum. The method of examination is similar to conventional upper GI endoscopy. Following the usual premedication, the sonoendoscope is introduced into the esophagus with the patient in the left lateral decubitus position. When the lesion is identified visually, it can be examined with the attached sonographic transducer. EUS-FNA has now become part of the diagnostic and staging algorithm for the evaluation of benign and malignant diseases of the GI tract and its bordering organs.1 Its uses within the GI tract include esophageal, gastric, duodenal, colon, and rectum. Lymph nodes related to lung cancer can be biopsied via the esophagus. Structures inferior to the esophagus and adjacent to the intestines such as pancreas, adrenal gland, gallbladder, bile duct, liver, kidney, and lung can be biopsied. Muscles of the inferior rectum and anal canal can be examined for fecal incontinence.
to the GI wall. An optical endoscope evaluates the lumen with a light. Endoluminal sonographic examinations are directed specifically at the bowel or surrounding structures. The coupling of sonography and endoscopy has allowed physicians to better visualize and stage cancers within or adjacent to the GI tract and to evaluate the depth of lesions within the intestinal wall. Transesophageal, transduodenal, and transgastric sonographic evaluations can detect abnormalities in the pancreatobiliary system, paraluminal and intramural lesions including lymphadenopathies, ascites, and lesions in the left liver lobe; and reasons for rectal bleeding, diarrhea, hematochezia, bowel obstruction, and change of bowel habit. Linear endoscopes have brought a new landscape to EUS because of their ability to track a needle in real time across the image plane into a target lesion. This is known as endoluminal ultrasound with fine needle aspiration (EUS-FNA). The electronic instruments also permit the use of Doppler technology to assess vascular flow.1 Endosonography has been performed primarily on the upper GI tract: the esophagus, stomach, and duodenum. The method of examination is similar to conventional upper GI endoscopy. Following the usual premedication, the sonoendoscope is introduced into the esophagus with the patient in the left lateral decubitus position. When the lesion is identified visually, it can be examined with the attached sonographic transducer. EUS-FNA has now become part of the diagnostic and staging algorithm for the evaluation of benign and malignant diseases of the GI tract and its bordering organs.1 Its uses within the GI tract include esophageal, gastric, duodenal, colon, and rectum. Lymph nodes related to lung cancer can be biopsied via the esophagus. Structures inferior to the esophagus and adjacent to the intestines such as pancreas, adrenal gland, gallbladder, bile duct, liver, kidney, and lung can be biopsied. Muscles of the inferior rectum and anal canal can be examined for fecal incontinence.
Endovaginal sonography (EVS), used to evaluate the prostate gland, is also suitable for evaluating the rectal wall and perirectal area and can be used to stage rectal tumors. Endovaginal transducers can locate and document the appendix in females and are especially helpful in the case of large body habitus.2 The same transducer visualizes portions of the inferior small intestine and colonic haustra.
Patient Preparation
Patient preparation for transabdominal sonography depends on the physician’s order. If the examination requisition for a GI ultrasound examination includes abdominal organs such as liver, gallbladder, pancreas, bile ducts, kidneys, and aorta, the patient should be advised to fast for 8 hours before the examination to ensure distention of the gallbladder and to minimize stomach gas. Morning studies are recommended to decrease intraluminal air, peristaltic motion, and patient hunger. The organs should be documented first, followed by the GI tract. Prior to examination of the stomach and duodenum, the patient can drink 10 to 40 oz of water through a straw to improve visualization. Having the patient drink fluid also helps in evaluating the mucosa and peristalsis of the jejunum and ileum. Oral fluid is contraindicated if the patient has GI obstruction or ileus or is scheduled for upper GI barium studies. Note that examinations can be performed in emergencies without preparation.
For transabdominal examination of the colon, no special preparation is necessary, except for the rare occasion during pelvic sonography when a water enema is required to establish the position of the rectosigmoid colon. Laxatives should be avoided prior to a GI ultrasound study. Osmotic laxatives cause water retention in the colon. Stimulant laxatives increase digestive movements. Both may contribute to abnormal sonographic results.
Before endorectal sonography, a cleansing enema may be helpful so that fecal material is not confused with mucosal lesions. EVS does not usually require bowel cleansing, although if the area of interest is obscured by intraluminal air or solid contents, a cleansing enema is recommended.
Limitations
Sonographic examination of the GI tract can be difficult because gas in the tract can obscure details of the bowel wall and lumen as well as structures deep into the bowel such as the pancreas and the periaortic area of the retroperitoneum or masses. Fluid or feces in the bowel lumen can be perceived as disease. Large patient body habitus may influence transducer selection. A high-frequency transducer should be utilized to obtain high-resolution images when possible. Obese patients often require a lower-frequency transducer for deeper penetration, which offers a lower-resolution image. Other limitations include the inability to continuously view the entire small bowel and, as with any sonographic examination, operator dependence.
Transducer Selection
Ultrasound examination of the GI tract usually benefits from the use of at least two different transducers. A low-frequency convex transducer in the range of 1 to 6 MHz is used to obtain initial GI views and for large habitus patients. A high-frequency linear probe ranging from 5 to 17 MHz will examine the bowel wall and evaluate superficial findings discovered with the low-frequency transducer. First, the abdomen is scanned by means of the convex low-frequency transducer, in order to visualize deeper structures and detect grossly abnormal pathologies, such as significant thickening of the intestinal wall, bowel dilatation, and the presence of fistulae or abscesses. This is followed by a linear higher-frequency transducer, for detailed evaluation of the intestinal wall, which requires extra time and patience by the ultrasound operator.3 Endovaginal and endorectal sonography are utilized to view layers of the rectum and stage rectal tumors, GI stroma tumors, inflammatory bowel disease, intestinal endometriosis, diverticulitis, appendicitis, bowel mass, and Crohn disease (CD) among others.
Patient Position
Examination of the intestinal tract begins with the patient comfortably relaxed in a supine position so as not to tense the abdominal wall.4 Flexing the left knee, especially when the knee is supported by a medical extremity wedge, alleviates back discomfort. The transducer is held maintaining contact with the patient’s skin to gauge pressure, while the
left hand is free to optimize image characteristics on the ultrasound system. A systemic approach in examining the whole intestine is encouraged.4
left hand is free to optimize image characteristics on the ultrasound system. A systemic approach in examining the whole intestine is encouraged.4
ANATOMY OF THE BOWEL WALL
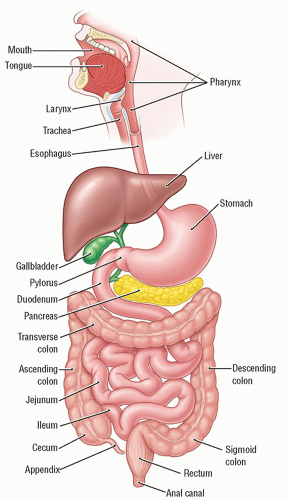
The GI tract is an approximately 30-foot muscular tube of varying diameter, originating at the lips and terminating at the anus (Fig. 11-1). The layers of the bowel wall are similar throughout the GI tract, with the esophagus having four layers and the rest of the GI tract having five layers.
Described anatomically, the innermost layer is the echogenic epithelium (mucosal surface). Within the esophagus, the innermost layer is composed of stratified squamous cells. In the stomach and bowel, this layer is composed of simple and columnar cells. Some of these cells are modified for digestive activities. The plasma membrane of the cells is adapted to perform certain functions: microvilli enhance the absorptive area, goblet cells secrete mucus, and cells with cilia oscillate to aid in digestion. Deep into the epithelium is a hypoechoic mucosal layer that consists of the lamina propria, which in the esophagus is a loose areolar tissue and elsewhere is a glandular tissue, and the muscularis mucosa, which is immediately below the lamina propria and is very thin and smooth. Just above and below the muscularis mucosa are scattered patches of lymphoid tissue. Below the muscularis mucosa is a looser areolar tissue, called the submucosa. It is the thickest layer and is echogenic. Deeper yet lies the hypoechoic muscularis propria, also known as the muscularis externa. This structure consists of an inner circumferential layer and an outer longitudinal layer of smooth muscle. The final layer of the wall is the echogenic serosa, a thin epithelial layer on the periphery of the bowel.
When using ultrasound frequencies in the range of 5 to 17 MHz, the wall of the intestine can be seen well and exhibits five different layers that correspond well to the histologic layers (Fig. 11-2A). The layered wall structure changes with disease.4 The five layers from interior to exterior are as follows:
an inner hyperechoic layer—commonly this represents the border between the digestive fluid and the mucosa;
a hypoechoic layer—usually thin, this represents the mucosa, lamina propria, and lamina muscularis;
a hyperechoic layer—the submucosa;
a hypoechoic layer—the muscular layer; its thickness depends upon the segment of the digestive tract; and
an outer hyperechoic layer—the serous layer, the border to the peridigestive fat.5
Thus, the sonographic structure of the bowel is usually described with five layers: three echogenic layers separated by two hypoechoic ones (Fig. 11-2B-D).5 Using high-frequency transducers, the muscularis mucosa and the two layers of the muscularis propria can be resolved as well. Visualizing the layers of the bowel wall is useful in detecting certain pathologic conditions that follow. A transabdominal approach demonstrates bowel wall layers with less definition than endoluminal sonography.
Gut Interrogation
GI ultrasound should examine the BWT, wall changes, vascular anomalies, motility, and symmetry of thickness. BWT is the measurement most consistently reported in diagnostic and activity trials.4 The measurement should include the outer hyperechoic layer to the inner hyperechoic layer and be performed with mild compression. Transducer compression of the bowel wall will reduce thickness and can make it challenging to distinguish wall layers. Some wall thickening is nonspecific, which may make it difficult or impossible to diagnose disease processes, although the finding must be evaluated. Focal, irregular, and asymmetrical thickening of the bowel wall suggests malignancy.6
ESOPHAGUS AND ESOPHAGOGASTRIC JUNCTION
Normal Anatomy of the Esophagus
The esophagus is a fibromuscular hollow tube positioned between the pharynx and stomach cardia within the thorax.
It is approximately 10 inches long. The superior width of the tube is 1.4 cm, with the distal width reaching 2 cm. Unlike the GI tract distal to the esophagus, it is composed of four layers: inner mucosa, submucosa, muscularis propria, and outer adventitia. It does not have a serosal layer. The mucosa is stratified squamous epithelium covering the entire lumen. The submucosa is a thick layer that connects the mucosa to the muscular layer. The muscular portion is made of longitudinal and circular muscle fibers. The outermost layer is adventitia composed of fibrous tissue that connects with the pharynx superiorly and with the stomach distally.
Sonographic Technique
A sonoendoscope is often utilized to assess the esophagus because this imaging method is able to view the entire lumen. Transabdominal sonography can depict most of the tubular structure in a supine patient using the thyroid as an acoustic window for the superior portion and the heart as an acoustic window with the transducer adjacent to the left sternum for the midportion. The inferior portion, esophagogastric junction, and stomach can be visualized transabdominally when using the left liver lobe as an acoustic window (Fig. 11-3A, B). The esophagogastric junction is within the superior abdomen (epigastric region) posterior to the left liver lobe and anterior to the aorta. An abdominal transducer should be placed inferior to the xiphoid and angled superiorly to locate the junction. Giving an erect patient water to drink may ensure transabdominal views of the entire esophagus. Layers of the esophageal wall and surrounding mediastinal structures will be seen best intraluminally. EUS provides an accurate evaluation of the depth of tumor invasion and the extent of lymph node involvement. PET is used to detect early esophageal cancer. MRI is adept at locating early esophageal lesions as well as detecting and staging wall invasion. CT detects lesions and can also search for spread of invasive disease. Barium swallow studies show esophageal lining anomalies, but are unable to determine the depth of wall invasion.
Sonographic Esophageal Wall Dimensions
Normal adult esophageal and esophagogastric junction single wall thickness is reported as 2 to 5 mm. Esophageal wall thickness may differ depending on contraction and dilatation. Acute conditions such as esophageal spasm, esophagitis, hematoma, and perforation can cause abnormal wall thickness measurement variations of the esophagus. Irregular measurements may be caused by a transient or acute condition. Focal causes for wall thickness include tumor, hernia, varices, and mucocele and are more likely to be long-lasting.
Disorders of the Esophagus
Carcinoma
Esophageal cancer is rare with an incidence of about 1% in the United States.7,8 The most common esophageal cancers are squamous cell carcinoma or, less commonly, adenocarcinoma per the Cleveland Clinic, Memorial Sloan Kettering Cancer Center, and the Mayo Clinic, among others. Squamous cell carcinoma is common in the upper and mid-esophagus, whereas adenocarcinoma mostly affects the distal portion. Both types affect men more than women, usually in those over 25 years of age (mostly over 65 years of age).7,8 The lesions begin as raised longitudinal plaque-like or polypoid areas, which quickly enlarge circumferentially; subsequently, strictures form and dysphagia develops. Because the esophagus lacks a serosa, extension into the surrounding mediastinum is unimpeded. Currently, about 20% of patients survive 5 years after diagnosis based on an average of localized, regional, and distant stages.7 Survival rates over 5 years are attributed to early detection.
Other Tumors of the Esophagus
Rare malignant tumors of the esophagus:
carcinosarcoma, mucoepidermoid carcinoma, adenoid cystic carcinoma
lymphoma
glomangiomas
Benign tumors of the esophagus:
polyp, granular cell tumor
adenoma
papilloma (malignant potential)
leiomyoma
Sonographic tumor findings:
wall deviations and disruptions
wall thickening
lesions with a polypoid, smooth-walled, vascular, or lobulated nature
mass with isoechoic, cystic, partially cystic, or heterogeneous appearance
Disorders of the Esophagogastric Junction
The esophagogastric junction can be evaluated with endosonography or, if the left lobe of the liver is large enough, with transabdominal sonography. The esophagogastric junction can be seen posterior to the left lobe and anterior to the abdominal aorta.5 A variety of abnormalities, including hiatal hernia, esophageal varices, anomalous motility, and tumors similar to those of the esophagus, can be demonstrated.
STOMACH
Normal Anatomy of the Stomach
The stomach is the expanded part of the alimentary tract and normally lies in the left upper quadrant of the abdomen. The fundus is medial to the spleen and anterior to the left kidney. The body and antrum of the stomach lie posterior or inferior to the left lobe of the liver, anterior to the pancreas, and medial to the gallbladder and porta hepatis.5 The antrum and body of the stomach often appear as a target-like structure inferior to the left lobe on longitudinal sonograms. Both hypo- and hyperechoic contents as well as air shadows can be seen (Fig. 11-4A-D). If the left hepatic lobe is large enough, the gastroesophageal junction can also be visualized as a target-like structure just below the diaphragm and just to the left of the spine. Gas, mucus, or
fluid may fill the center of the stomach (Fig. 11-5). Often, when fluid is present in the stomach, the rugal folds and posterior wall structure can be seen.
fluid may fill the center of the stomach (Fig. 11-5). Often, when fluid is present in the stomach, the rugal folds and posterior wall structure can be seen.
Sonographic Technique
Within the GI tract, the stomach has the most potential for a sonographic diagnosis. The layers of the GI tract wall are thicker here than anywhere else and nearly always can be visualized transabdominally in the normal patient. A few special techniques are occasionally helpful. If uncertainty exists whether a cystic structure in the left upper quadrant represents the stomach, giving the patient a few sips of water through a straw produces a sparkling or swirling pattern in the stomach as the water flows. A recommendation to view the stomach also includes giving the patient 500 to 800 mL (approximately 20 to 30 oz) of plain water to allow filling of the cavity followed by sonographic exploration 10 to 15 minutes after the water ingestion.5 This allows air bubbles to diffuse from the water, which removes artifact that may be suspicious of disease. Usually, the stomach contains some air when the patient is supine. Follow the suggested transducer positions in Figure 11-6 to examine the stomach.
Placing a patient in the right lateral decubitus position moves fluid into the antrum and pyloric region of the stomach, as well as the duodenum, which provides better visualization. Some studies suggest that the antrum and body are viewed best with a semi-erect patient. Turning the patient into the left lateral decubitus position often improves visualization of the fundus.
If a solid-looking mass is suspected to be in the stomach, or when detailed evaluation of the gastric mucosa is required, the patient should be examined first with minimal stomach contents and again after water ingestion in the upright, left lateral decubitus, supine, and right lateral decubitus positions, thus demonstrating most of the gastric mucosa. Some investigators recommend giving 1 mg of glucagon intravenously before the patient drinks the water to ensure retention of the fluid in the stomach—this should produce 30 to 60 minutes of gastric distention.
 FIGURE 11-6 The illustration displays the recommended transducer position to acquire the best sonographic visualization of the stomach. |
Examination of the stomach can be done with a 3.5 or 5 MHz transducer. The gastric wall should be examined with a 5 or 7.5 MHz transducer. The examination should begin at the distal esophagogastric junction at the level of the cardia portion of the stomach, moving into the fundus, body, antrum (most consistently identified portion), pylorus, and then duodenum. Use the left lobe of the liver to identify the esophagogastric junction, then move toward the spleen intercostally to identify the cardia and fundus. A second option is to scan sagittally near the midaxillary line superior to inferior. Note that the cardia and fundus are deep and that air is usually positioned in this region, as well as the body of the stomach, causing limitations. The stomach body is visualized inferior to the left anterior ribs. The anterior wall is usually seen, although the posterior wall may be obscured by air. Examine the antrum with the patient in a supine position. It is viewed well sagittally adjacent to the left liver lobe and the pancreas. Again, with the patient in a supine position, the pylorus is located by finding the gallbladder, then adjusting the transducer in an oblique fashion longitudinally. The pylorus is located medial and posterior to the gallbladder.
Upper GI radiographic studies and EUS are commonly performed to search for anomalies of the stomach walls and its function. Gastric ultrasound via the abdominal method displays differing appearances depending on fasting and ingestion of fluid versus solids. The antrum can appear flat to oval in shape with a fasting patient. Upon fluid ingestion, a glittery appearance will be seen as the fluid and air whirl in the stomach lumen. Once the fluid separates from the air, a hypoechoic interface will be seen with air (brighter), and the interface will change when the patient moves to different positions. Ring-down artifacts will be visualized when air moves within the stomach. Food in the stomach lumen appears similar to a whiteout snowstorm. CT, MRI, and PET are not routinely used imaging modalities for gastric assessment.
Sonographic Stomach Wall Dimensions
If the stomach is not distended, its wall should measure approximately 3 to 6 mm thick. When the stomach is distended to a diameter of 8 cm or more, the wall should measure 2 to 4 mm. Bowel abnormalities and thickening are caused by infiltrative and inflammatory processes, infection, edema, ischemia, or neoplastic invasion. In general, the criterion for abnormal thickening of the gastric wall is a thickness of
greater than 5 mm.6 The wall is also considered abnormal if a normal-appearing portion is visualized adjacent to a significantly thicker section.
greater than 5 mm.6 The wall is also considered abnormal if a normal-appearing portion is visualized adjacent to a significantly thicker section.
Disorders of the Stomach
Gastric Dilatation
Many disorders can cause the stomach to dilate (see Fig. 11-5). Gastric outlet obstruction (a dilatation) is a clinical syndrome that can manifest with a variety of symptoms, including abdominal pain, postprandial vomiting, early satiety, and weight loss. It is caused by either a benign or malignant mechanical obstruction or a motility disorder interfering with gastric emptying. Anatomically, the mechanical obstruction can be at the distal stomach, pyloric channel, or duodenum, and it can be intrinsic or extrinsic to the stomach.9 Acute dilatation can occur after surgery, because of adhesions blocking the small bowel, tumor, ulcer, pyloric muscle hypertrophy, or after placement of a body cast. It is not clear whether this dilatation is caused by reflex paralysis of gastric motility or obstruction of the duodenum by the superior mesenteric artery impinging on it. Tumor or ulcers may obstruct the gastric outlet. Pyloric muscle hypertrophy is rare in adults, but when it does occur, it is usually associated with gastritis or ulcer. Other factors contributing to dilatation of the stomach are diabetes mellitus, scleroderma, or surgical vagotomy, which may bring about gastric dilatation as a complication of neuropathy. Observation of gastric peristalsis can be made using an abdominal imaging approach to differentiate atonic from obstructive dilatation, but the distinction may be impossible in many cases because of rigidity of the stomach wall, often seen in tumor infiltration and gastric ulcer disease and the uncoordinated peristaltic waves seen in neuropathic conditions. Volvulus is another rare cause of gastric dilatation.
Gastritis
Gastritis is inflammation of the gastric mucosa caused by several conditions, including infection, drugs, stress, and autoimmune phenomena.10 It can be classified based on the site of involvement and as acute or chronic. Chronic gastritis is caused by several factors and may present as enlarged rugal folds with generalized thickening of the mucosal layer of the wall (Fig. 11-7A, B). This thickening may accompany either increased acid production, as in Zollinger-Ellison syndrome, or decreased acid production, as in Ménétrier disease. Chronic gastritis may also demonstrate hyperplastic and inflammatory polyps. Another variation is atrophic gastritis, in which the mucosa is thinned. This may be difficult to see sonographically, but it is considered a precursor of gastric carcinoma.
Ulcer Disease
Peptic ulcer disease (PUD) is characterized by discontinuation of the inner lining of the GI tract because of gastric acid secretion or pepsin. It extends into the muscularis propria layer of the gastric epithelium. It usually occurs in the stomach and proximal duodenum. It may involve the lower esophagus, distal duodenum, or jejunum.11 The ulcers mostly appear along the antral portion of the lesser curvature. Sonographically, there may be major wall thickening, usually caused by marked edema of the submucosa, with milder thickening of the gastric mucosa (Fig. 11-8A, B). Typically, the mucosa is undercut at the edge of a benign ulcer. A malignant ulcer, by contrast, should show more “heaping up” of the margin of the ulcer. The bowel layers adjacent to the ulcer may be obliterated in either benign or malignant ulcers. Ulcers can be detected sonographically, particularly if they are large, but often, they are only suggested by focal or generalized edema of the wall. EUS can detect defects, such as ulceration, in the gastric wall required to diagnose superficial ulcerations of the mucosal lining, as well as behind the base of the defect. It is helpful in differentiating benign from malignant gastric ulcers. CT of the abdomen with contrast is of limited value in the diagnosis of PUD itself but is helpful in the diagnosis of its complications like perforation and gastric outlet obstruction.11
Complications of PUD, gastric or duodenal, include anterior or posterior perforation. Usually, anterior perforation results in free intraperitoneal air and, often, subsequent peritonitis, which may appear sonographically as ascites, loculated ascites, or dense debris in the peritoneal space. Some reports describe the appearance of free air in the peritoneal region as an increase in echogenicity of a peritoneal stripe with multiple reflective artifacts and a comet tail appearance. Care must be taken to differentiate this from bowel gas. Thickening of the bowel serosa may be observed because of peritoneal irritation and reactive edema. Generalized peritoneal infection or localized abscess may also result. The chief complication of posterior duodenal perforation is
bleeding. Bleeding from an ulcer can be slow and go unnoticed or can cause life-threatening hemorrhage. Ulcers that bleed slowly might not produce symptoms until the person becomes anemic. Bleeding that occurs more rapidly might show up as melena, a jet black, very sticky stool, or even a large amount of dark red or maroon blood in the stool. People with bleeding ulcers may also vomit. This vomit may look like red blood or “coffee grounds.”12 Other symptoms might include “passing out” or feeling light-headed. Posterior perforation of the stomach may result in pancreatitis.
bleeding. Bleeding from an ulcer can be slow and go unnoticed or can cause life-threatening hemorrhage. Ulcers that bleed slowly might not produce symptoms until the person becomes anemic. Bleeding that occurs more rapidly might show up as melena, a jet black, very sticky stool, or even a large amount of dark red or maroon blood in the stool. People with bleeding ulcers may also vomit. This vomit may look like red blood or “coffee grounds.”12 Other symptoms might include “passing out” or feeling light-headed. Posterior perforation of the stomach may result in pancreatitis.
Gastroduodenal Crohn Disease
CD is an idiopathic inflammation that starts in the submucosa and spreads to all layers of the bowel wall. This disease is chronic and usually occurs in young adults. Although this disease may affect any part of the GI tract, the terminal ileum is the most common site.13 It is rare in the stomach and duodenum; approximately 2% to 8% of patients have stomach or duodenal involvement. Because the entire wall is involved at diagnosis, the sonographic appearance is that of a nonspecific hypoechoic target lesion if the lumen is viewed transversely (Fig. 11-9A-E). Chronic inflammation is seen as hypervascular echoes in the wall. Followup ultrasound examinations may be used often for patients diagnosed with CD. Continued interrogation of the GI walls for abscess and thickness are necessary during and following treatment of the condition. Advanced carcinoma, lymphoma, hematoma, abscess, colitis, and tuberculosis can appear similar to CD (Fig. 11-10A-D).
Other Inflammatory Conditions
Infection of the stomach can display marked thickening of the stomach wall and swelling of the gastric rugae, a condition called phlegmonous gastritis. Most cases are caused by
bacteria such as α-hemolytic streptococci; Staphylococcus, Escherichia coli, Clostridium welchii, H. pylori, and Proteus species have also been found. Peritonitis occurs in 70% of cases. When gas-forming organisms such as E. coli or C. welchii are the cause, small gas bubbles may form in the gastric wall—this is a type of emphysematous gastritis. Swallowing a corrosive substance is a more common cause of emphysematous gastritis.
bacteria such as α-hemolytic streptococci; Staphylococcus, Escherichia coli, Clostridium welchii, H. pylori, and Proteus species have also been found. Peritonitis occurs in 70% of cases. When gas-forming organisms such as E. coli or C. welchii are the cause, small gas bubbles may form in the gastric wall—this is a type of emphysematous gastritis. Swallowing a corrosive substance is a more common cause of emphysematous gastritis.
Gastric Cancer
Gastric or stomach cancer is an inclusive term for common cancers affecting the stomach. The incidence of gastric cancer has declined in the United States and represents approximately 1.5% of all new U.S. cancer cases detected annually.14 The 5-year survival rate for localized gastric cancer (no cancer spread outside the stomach) is 69% and the 5-year survival rate for regional gastric cancer (cancer spread to nearby structures/lymph nodes) is 31% in the United States.15 Stomach cancer is much more common in other parts of the world, particularly in less developed countries. It is the fifth most common neoplasm and the third most deadly worldwide.
Adenocarcinoma
Adenocarcinoma is the most common type of cancer of the stomach. It accounts for 90 to 95% of gastric cancers.16 This cancer type originates in the glandular tissue known as epithelial tissue. The stomach is lined with a mucous membrane composed of columnar epithelial cells and glands. Gastric carcinoma invades the submucosa and muscularis propria (Fig. 11-11A, B). These cells are prone to inflammation, known as gastritis, which can lead to peptic ulcers and, ultimately, gastric cancer. Expect sonographic appearance to demonstrate hypoechoic localized or diffuse thickening of the walls because of the invasion by the cancer or polypoid lesions.
Gastric adenocarcinomas are primarily classified as cardia and noncardia based on their anatomic site; they arise in the region adjoining the esophageal-gastric junction and, thus, share epidemiologic characteristics with esophageal adenocarcinoma. Noncardia cancer, also known as distal stomach cancer, is more common and arises in the lower portion of the stomach.16 Most gastric cancers spread primarily toward the serosa of the gastric wall. Some gastric tumors arise in the margin of a long-standing benign peptic ulcer.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree