Fig. 10.1
Distal radial buckle fracture in a 6-year-old boy. (a, b) There is angulation of the distal radial metaphyseal cortex (arrows)
Bicortical and more complex fractures are the result of bending—rotation or shear—and involve both cortices [5]. With regard to displacement, in general, angulation of less than 20° in the anteroposterior (AP) plane and 10° in the transverse plane is considered adequate for remodeling [5]. The remodeling potential of the fracture will be greatest in young children, in fractures that are close to the physis, and in fractures which are angulated in the AP plane. Rotational misalignment will not remodel [5]. Depending on the alignment, reducibility, and the presence of associated neurovascular injuries, these fractures may be treated with closed reduction and casting, open reduction, or surgical fixation.
Unique to pediatrics, involvement of the physis does sometimes precipitate disturbed growth. Approximately 20 % of distal radial fractures involve the physis (most commonly Salter-Harris type II fractures) [5]. However, the risk of physeal growth arrest for a distal radial fracture is relatively small, approximately 4 % [7]. A smaller proportion of distal ulnar fractures involve the physis, but the risk of physeal growth arrest is high—up to 50 % [7].
Imaging
Radiographs are generally sufficient for imaging distal radial and ulnar fractures. With a buckle fracture, radiographs demonstrate angulation of the cortical margin, commonly at the metaphysis. By definition, the opposite cortex is intact and the degree of displacement minimal. Adjacent soft tissue swelling, evidenced by obscuration of the fat planes, is usually present. The most easily visualized fat plane is the pronator fat pad, located volar to the distal radial and ulnar metaphyses. The pronator fat pad (Fig. 10.1) often becomes indistinct or completely obscured when there is significant soft tissue edema. Bicortical fractures manifest radiographically with or without displacement of the fracture fragments. Distal radial fractures commonly have an associated distal ulnar fracture, often involving the styloid process. Computed tomography (CT) or magnetic resonance imaging (MRI) is used for more complex fractures or if there is concern for soft tissue impingement and other complications.
1.2 Carpal Bone Fractures
Carpal bone fractures are infrequent in children, accounting for less than 1 % of all pediatric fractures [8, 9]. One proposed explanation is that the non-ossified cartilage surrounding the ossification centers of the carpal bones acts as a shock absorber, protecting the ossification center from impact and fractures [10]. The incidence of carpal bone fractures is highest in the teenage years and is extremely low in patients under age 8 [9].
Scaphoid Fractures (Box 10.1)
Box 10.1: Scaphoid Fractures
Most common carpal fracture (but unusual in children) |
Low incidence of nonunion in children |
Scaphoid waist is the most often fractured |
Fractures of the scaphoid waist and especially the proximal pole most often lead to distal avascular necrosis |
In children as in adults, the scaphoid is by far the most commonly fractured carpal bone [9, 11]. For example, in one series, scaphoid fractures accounted for approximately 89 % (33/37) of carpal fractures [9]. The peak incidence of scaphoid fractures is between the ages of 11 and 15 [9, 12]. Patients typically present with a history of fall on outstretched hand and pain localized to the anatomic snuffbox.
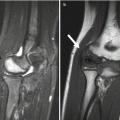
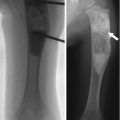
In the past, scaphoid fractures in children differed from those in adults, more likely to occur at the distal third of the scaphoid [9, 11, 12], possibly due to early ossification of the distal scaphoid and the protective effect of thicker soft tissues over the proximal pole [11] (Fig. 10.2). However, this pattern appears to have changed, with altered patient characteristics and activities. Acute scaphoid fractures in children now most often involve the scaphoid waist, as in adults [13] (Fig. 10.3). Most fractures are non-displaced, and incomplete fractures also occur [8]. Nonunion is less common in appropriately treated pediatric patients than in adults [9]. Since most of the blood supply to the scaphoid is distal, transverse fractures of the proximal or mid-scaphoid often result in avascular necrosis of the distal pole.
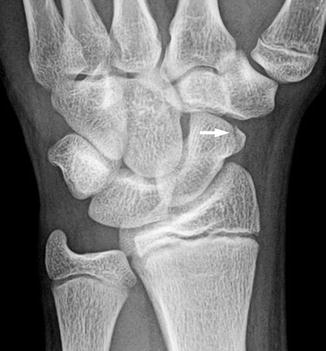
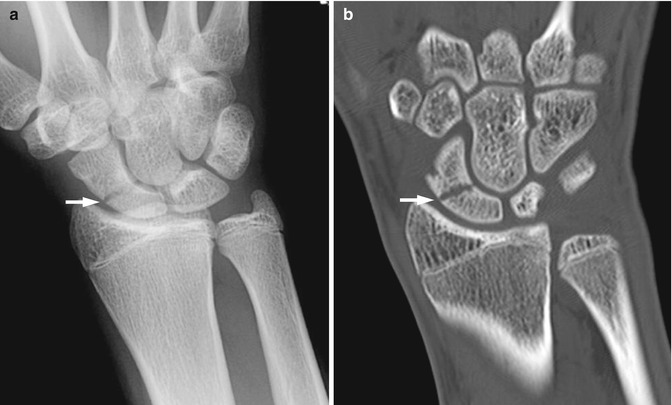

Fig. 10.2
Fracture through distal scaphoid in a 16-year-old boy (arrow)

Fig. 10.3
Scaphoid fracture in a 17-year-old boy. (a) Transverse fracture through the waist of the scaphoid (arrow). (b) CT confirms transverse fracture (arrow)
Scaphoid fractures in the acute setting are usually immobilized with either a short- or long-arm thumb-spica cast [8, 9]. In rare cases with significant displacement, acute operative management is required. More often, surgical treatment is reserved for cases of nonunion after failed nonoperative management. The relatively low incidence of nonunion, coupled with the more distal location of the fractures, makes the serious complication of avascular necrosis of the proximal pole of the scaphoid rare [14].
Imaging
If there is suspicion of a scaphoid fracture, a dedicated “scaphoid view” with the hand in ulnar deviation should be included, along with standard hand or wrist radiographs. CT may be helpful in selected cases to confirm the presence of a fracture. MRI has been shown to be more sensitive than radiographs in equivocal or clinically discrepant cases [15]. Findings on MRI include focal bone marrow edema, a low signal fracture line on T1-weighted (T1-W) images, and cortical disruption [15].
In the non-acute setting, CT is useful to evaluate nonunion and for subtle evidence of healing. MRI is helpful for suspected cases of avascular necrosis. Findings include low signal on both T1- and T2-weighted (T2-W) images and a lack of enhancement after intravenous contrast administration.
Other Carpal Bone Fractures
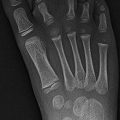
Fractures of the other carpal bones are extremely rare in children. Fractures of the capitate, triquetrum, hamate, trapezoid, and pisiform have been reported [16] (Figs. 10.4 and 10.5). Most often, these fractures are the result of a fall or a direct blow [9, 16]. Carpal fractures are typically managed nonoperatively and have an excellent prognosis [9].
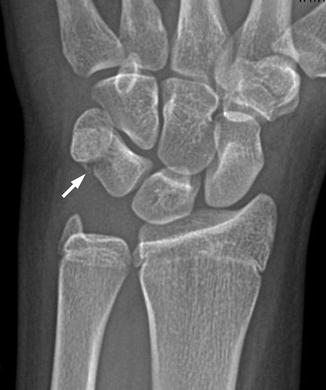
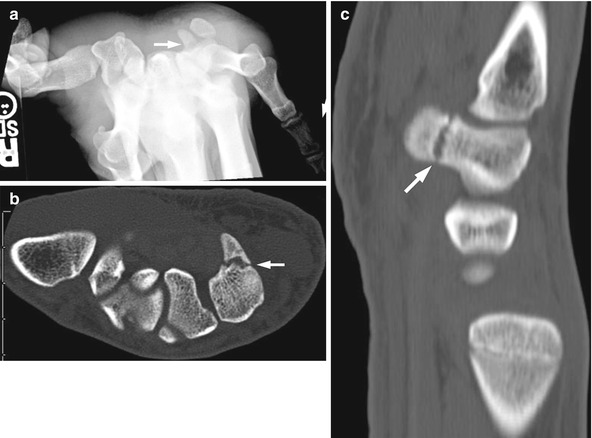

Fig. 10.4
Triquetral fracture in a 15-year-old girl that occurred during a softball injury. There is focal disruption of the triquetrum (arrow)

Fig. 10.5
Fractured hook of the hamate in a 17-year-old hockey player injured 2 weeks earlier. (a) Carpal tunnel view demonstrates questionable lucency through the hook of the hamate. Axial (b) and sagittal reformatted (c) CT images demonstrate a non-displaced fracture of the hook of the hamate (arrows). Sclerosis along the fracture line suggests the injury is beginning to heal
Imaging
The most common radiographic findings are a lucent fracture line or cortical disruption. Some fractures have more unique patterns; for example, a fracture of the triquetrum often manifests as a small osseous fragment posterior to the proximal carpal row on the lateral radiograph [16]. Most fractures do not demonstrate significant displacement [9]. MRI can be used in difficult cases and is more sensitive to bone marrow edema and non-displaced fractures [16]. Findings on MRI include increased T2 signal in the bone marrow, cortical disruption, and visualization of a discrete low T1 signal fracture line.
1.3 Hand Fractures
The hand is the most commonly injured part of the pediatric skeleton [11, 17, 18]. The phalanges are more frequently injured than the metacarpals. Injuries are also more common in the so-called border digits—the thumb and fifth finger [17, 18]. Fractures of the hand are most common in older children with a reported peak incidence between ages of 9 and 13 years [17, 18]. The radiographic evaluation of suspected fractures of the hand should include posteroanterior (PA), oblique, and lateral projections with the fingers fanned.
Metacarpals
Although fractures of the metacarpals usually involve the shaft or neck, periarticular and periphyseal fractures are also relatively frequent [11]. Metacarpal fractures typically occur as the result of a crush injury or a compressive force, such as punching a hard object (Fig. 10.6). The first and fifth metacarpals are most frequently injured [17]. Radiographs are usually sufficient for diagnosing a metacarpal fracture. The degree of displacement and angulation across the fracture site varies with mechanism of injury. Treatment depends on the degree of displacement and any associated injuries. Most fractures are treated with immobilization, although some require closed reduction or even surgical management. The prognosis is generally excellent.
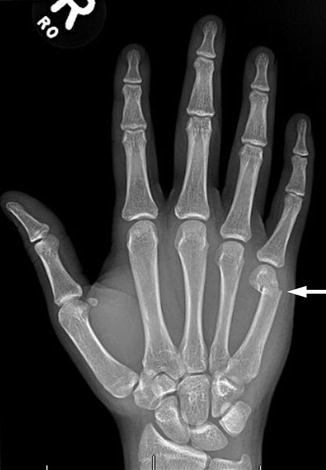

Fig. 10.6
Fifth metacarpal fracture after a 16-year-old boy punched a wall. Note the characteristic apex dorsal angulation (arrow)
Phalanges
The majority of phalanx fractures are periphyseal or periarticular [18]. The most common is a Salter-Harris type II fracture of the proximal phalanx [17–19]. This type of injury is often secondary to rotation or shearing [18]. Buckle fractures of the phalangeal metaphyses are also common. Fractures of the phalangeal neck are unique to children and can be difficult to visualize on radiographs.
Distal phalangeal fractures are usually secondary to a crush injury. In younger children, this usually results from an accident, such as catching a finger in a closing door [18]. In older children, these injuries are often related to participation in sports [17]. Crush injuries that involve the nail bed may require prophylactic antibiotics due to a relatively high incidence of osteomyelitis [11]. Of special importance is the Seymour fracture, a physeal fracture of the distal phalanx which is open through the nail bed. Clinically this will present as a subungual hematoma with a displaced physeal fracture on radiographs. Removal of the nail plate and irrigation of the fracture are required to prevent infection.
In general, the treatment of most phalangeal fractures is nonsurgical. In one series, almost 90 % were managed solely by immobilization. Surgical treatment was reserved for fractures which were unstable or had significant intra-articular involvement, rotation, or shortening [18]. The prognosis for most phalangeal fractures is excellent. Despite the periphyseal predominance, associated growth disturbances are rare [17].
Imaging
Radiographs typically demonstrate a lucent fracture line. Incomplete and buckle fractures also occur. The degree of displacement varies with the mechanism of injury, although two-thirds are displaced either minimally or not at all [17]. Cross-sectional imaging with CT or MRI is reserved for cases with complex fracture patterns or when associated soft tissue injury is suspected. Salter-Harris II fractures are the most common, although other Salter-Harris fractures also occur [18]. Ligament or tendon injury can be associated with Salter-Harris III fractures [11]. For example, mallet finger occurs with a fracture of the base of the distal phalanx and disruption of the extensor digitorum tendon, resulting in the inability to extend the finger at the distal interphalangeal joint. Another specific injury that deserves mention is a Salter-Harris III fracture of the proximal phalanx of the thumb. As in adults, this fracture can be associated with ulnar collateral ligament injuries and may require surgical correction [11].
2 Kienböck Osteonecrosis (Box 10.2)
Box 10.2: Kienböck Osteonecrosis
Associated with negative ulnar variance |
Four radiographic stages |
I = normal |
II = sclerosis |
III = lunate collapse |
IV = carpal instability |
MRI most sensitive for early stages |
Kienböck osteonecrosis (also known as Kienböck disease or malacia, lunatomalacia, and osteonecrosis of the lunate) was first described by the Austrian radiologist Robert Kienböck in 1910. Commonly seen in young adults, the peak age incidence is 20–40 years [20]. The disorder does occur in pediatric patients, usually in mid to late adolescence [21, 22]; however, it has been reported in patients as young as 6 years of age [23].
The etiology is unknown and thought to be multifactorial, but the most widely accepted theory is that vascular perfusion of the lunate is ultimately altered [24]. One potentially important anatomic consideration in Keinböck disease is that of negative ulnar variance. Negative ulnar variance describes a relative foreshortening of the ulna in relation to the radius. Some studies have shown a correlation between negative ulnar variance and an increased incidence of Kienböck disease, but others have shown no association [20]. It is proposed that negative ulnar variance alters the mechanics of the radiolunate joint and results in increased mechanical stress. Interestingly, this association has not been found in patients with negative ulnar variance after ulnar shortening procedures [20].
The treatment of Kienböck disease varies with patient age, severity of symptoms, and the extent to which function is impaired. Various treatments, including supportive measures, ulnar lengthening, radial shortening, revascularization of the lunate, and arthrodesis with carpal fusion have been employed. The most commonly described treatments in children are supportive therapy and radial shortening surgery [21, 22, 25]. With treatment, the radiographic abnormalities of the lunate may completely normalize [25].
Imaging
Radiographs demonstrate varying degrees of lunate abnormality, ranging from mild to complete collapse; with advanced disease, secondary degenerative changes may develop. Lichtman radiographic staging system is most widely used and classifies the appearance as I (normal), II (sclerosis), III (lunate collapse), and IV (carpal instability) [24]. MRI is more sensitive to early stages of Kienböck disease [26, 27]. Early disease (Lichtman stage I on radiographs) manifests as decreased T1 signal and increased T2 signal, reflecting bone marrow edema. The imaging appearance at this stage is nonspecific, resembling edema secondary to trauma. As the disease progresses (Lichtman stage II on radiographs), there will be low signal on both T1- and T2-W images, reflecting increased sclerosis [26]. More advanced stages of the process (Lichtman stages III and IV on radiographs) will manifest as diffuse T1 and T2 hypointense signal changes throughout the lunate, coupled with alterations in lunate shape (i.e., collapse) and bone marrow edema in the adjacent carpus. CT, with its excellent spatial resolution, can be a useful tool both for evaluating subtle findings of sclerosis and collapse and for evaluating associated perilunate degenerative changes [26] (Fig. 10.7).
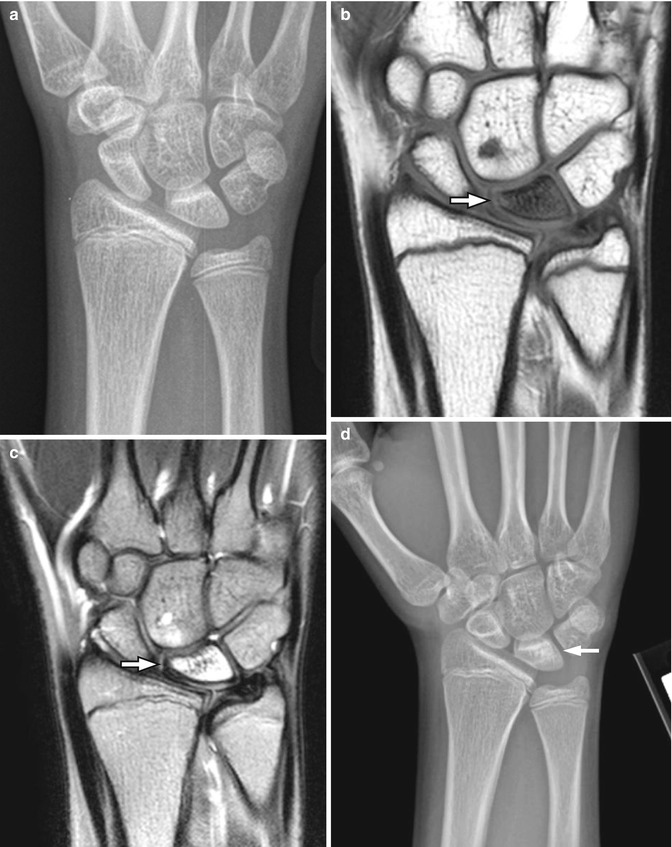

Fig. 10.7
Keinböck osteonecrosis in a 13-year-old girl. (a) Initial radiographs were normal except for negative ulnar variance. Coronal T1-W (b) and T2-W fat-suppressed (c) images show diffuse low T1 signal and corresponding high T2 signal (arrows). Similar abnormal marrow signal in the capitate is likely secondary to altered mechanical stress related to the abnormal lunate. (d) One month later, there is sclerosis and mild collapse of the lunate
3 Overuse Injuries
3.1 Gymnast Wrist (Box 10.3)
Box 10.3: Gymnast Wrist
Overuse leads to chronic physeal injury | |
Radius much more common and severe than the ulna | |
Distal physeal widening, irregularity, and haziness | |
Cystic change in distal metaphysis | |
Premature closure of distal physis | |
MRI | T2 hyperintense cartilage extends into metaphysis |
Edema, premature physeal closure, and bone bridging | |
Growing bones are uniquely susceptible to injury from repetitive stress or loading. For example, injury to the distal radial physis occurs in young gymnasts. Wrist pain is common in competitive and elite gymnasts, with an estimated prevalence of 46–79 % [28, 29]. Up to half of beginner or mid-level gymnasts report wrist pain as well [30].
Due to the nature of several of the maneuvers performed in competitive gymnastics, the wrist, which is normally not a weight-bearing structure, commonly supports the athlete’s entire weight [28]. The distal radius, in particular, bears the brunt of the injury as it supports approximately 80 % of the wrist’s axial load [31, 32]. In younger children, in whom there is typically a degree of negative ulnar variance [33], the radius can be called upon to support up to 96 % of the load [32].
Chronic mechanical stress decreases the vascular supply to the metaphysis, inhibiting ossification of chondrocytes at the zone of provisional calcification [34, 35]. The radiographic findings of growth plate widening and cystic changes in the metaphysis result from the accumulation of these non-ossified chondrocytes at the metaphyseal margin of the growth plate and within partially ossified portions of the metaphysis. This correlates with the histopathological findings seen in animal models [35]. Excessive mechanical stress to the physis has also been linked to premature physeal closure [36]. Stress fractures of the distal radial metaphysis can also be seen. Occasionally, in the setting of an acute injury, a Salter-Harris type I fracture of the distal radius with physeal widening and displacement of the epiphysis may occur.
A clinical and radiographic correlation has been found between gymnasts and positive ulnar variance, or relative increased length of the ulna in relation to the radius. As mentioned above, negative ulnar variance occurs in the skeletally immature wrist [33]. However, in competitive gymnasts, even those that have not yet reached skeletal maturity, there is an increased rate of positive ulnar variance [37, 38]. This may be secondary to growth disturbance of the distal radius resulting in relative overgrowth of the ulna. However, no direct evidence links distal radius stress and growth disturbance to wrist pain and positive ulnar variance [32].
Clinical management of a young gymnast with wrist pain is generally supportive. Imaging is usually performed in order to characterize the injury and to exclude acute fractures or soft tissue injuries. In general, treatment consists of rest and cessation of load-bearing activities. Gradual return to activities is recommended, with routines altered to minimize the load on the wrists [32]. The efficacy of bracing, either prophylactically or as treatment, has not been well established [32].
Imaging
Radiographic findings in these patients vary. In some patients, radiographs are normal; however, the distal radial physis is often widened [39]. Additional radiographic findings may include cystic changes in the distal radial metaphysis, beaking of the epiphysis, and haziness of the normally radiolucent physis (Fig. 10.8) [40]. Premature closure of the distal radial physis has also been reported [41]. Pseudo-Madelung appearance has been described, secondary to premature closure of the ulnar aspect of the radial physis [42]. Acute Salter-Harris type I fractures manifest as displacement of the epiphysis in relation to the metaphysis. In patients undergoing MRI, findings include physeal widening, abnormal T2 hyperintense cartilage extending proximally into the distal radial metaphysis, T2 hyperintense bone marrow edema, and premature physeal closure with bridging (Fig. 10.9) [43]. Similar changes can also be seen in the ulna but are less common and are often less pronounced [39, 43, 44].
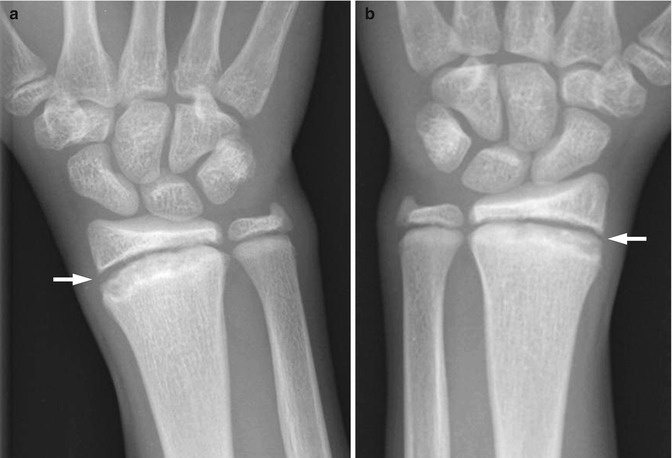
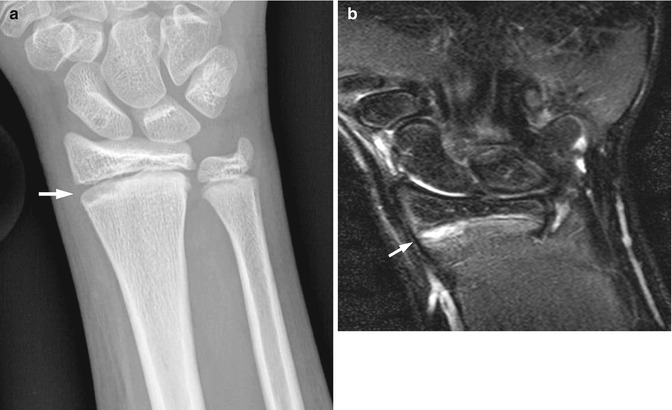

Fig. 10.8
Gymnast wrist in a 13-year-old girl. (a, b) There is bilateral metaphyseal irregularity and sclerosis with widening of the distal radial physes

Fig. 10.9
Gymnast wrist in a teenager. (a) Asymmetric widening of the distal radial physis (arrow). (b) Coronal short tau inversion recovery MRI shows corresponding increased thickness and high T2-W signal at distal radial physeal cartilage (arrow), with adjacent marrow edema (Courtesy of Diego Jaramillo, Philadelphia, PA)
3.2 Ulnocarpal Impaction (Box 10.4)
Box 10.4: Ulnocarpal Impaction
Degeneration due to abutment of distal ulna into proximal carpal row |
Early findings: Radiographs–usually normal MRI–T1 hypointense and T2 hyperintense signal (edema) within lunate and occasionally within triquetrum |
Late findings: Radiographs–lunate sclerosis and (with advanced disease) collapse and fragmentation; secondary degenerative changes of the carpus. MRI–T1 and T2 hypointense signal in lunate (sclerosis), along with fragmentation and collapse |
Patients with ulnocarpal impaction syndrome present with slow-onset wrist pain exacerbated by pronation and ulnar deviation. Causes include congenital ulnar positive variance, trauma that affects relative growth of the distal radius or ulna, Madelung deformity, and elbow trauma [45]. Abutment of the distal ulna into the proximal carpal row causes degeneration.
Treatment for ulnocarpal impaction syndrome is based on the patient’s symptoms and can be conservative or surgical [45]. Corticosteroid injections and nonsteroidal anti-inflammatory medications can provide symptomatic relief. Surgical options include either ulnar shortening osteotomy or a wafer procedure, which removes a portion of the ulnar head while preserving the radioulnar ligaments, fovea, triangular fibrocartilage complex (TFCC), and articular surface of the distal radioulnar joint (DRUJ) [45].
Imaging
Early MRI shows ulnocarpal impaction syndrome while radiographs are still normal (Fig. 10.10). Edema is seen in the lunate, most commonly followed by edema within the radial portion of the triquetrum. According to one study, only 10 % of patients demonstrated edema within the ulnar head [46]. Late MRI shows decreased signal intensity on T1- and T2-W images, suggesting subchondral sclerosis; radiographs show sclerosis of the involved osseous structures and subchondral cystic change. Ulnar impaction can also result in degeneration of the TFCC. This can progress to complete tears, best identified with MR arthrography.
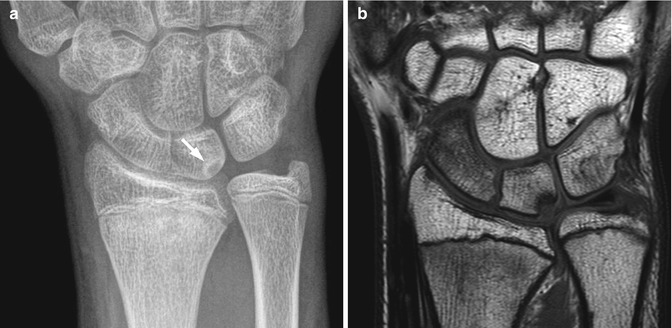

Fig. 10.10
Ulnocarpal impaction in a 14-year-old girl. (a) There is positive ulnar variance and cystic change along the ulnar aspect of the lunate (arrow). (b) Coronal proton density MRI confirms ulnocarpal impaction
Differential Diagnosis
Differential diagnosis of ulnar impaction syndrome includes other causes of ulnar-sided wrist pain including TFCC tears and lunotriquetral ligament injury.
4 Pathology of Tendons and Ligaments
4.1 Tendons
Overuse injuries such as tendonosis are generally more common in adolescents than in younger children. Risk factors include a change in training intensity, muscle-tendon imbalance, poor conditioning prior to training, nutritional deficiencies, and rapid growth [47, 48]. Tendonosis and tendon injuries typically occur with repetitive motion or impact, such as in gymnastic and racquet sports. The most common tendon pathology in the wrist occurs in the first and sixth extensor compartments.
First Extensor Compartment
de Quervain tenosynovitis is a stenosing tenosynovitis involving the first extensor compartment of the wrist. It presents with pain and functional impairment. Generally, females are more affected than males. This condition is more commonly seen in active children.
This tenosynovitis is caused by repetitive microtrauma at the level of the extensor retinaculum. Inflammation of the tendon sheaths of the extensor pollicis brevis (EPB) and abductor pollicis longus (APL) results in fibrotic changes [49]. There is anatomic variability in the first dorsal compartment tendons—some people have more than one slip of the APL tendon, while some may also have a separate tendon sheath for the EPB. These anatomic differences may increase the risk of developing de Quervain.
Ultrasound has been used to guide corticosteroid injections into the tendon sheath, resulting in decreased fluid within the sheath as well as rapid clinical improvement [50, 51].
Imaging
Although the diagnosis of de Quervain tenosynovitis is mostly clinical, both ultrasound (US) and MRI can be used to investigate this condition. In acute cases, US demonstrates fluid surrounding the tendon, causing distension of the tendon sheath; the synovial fluid appears as a hypoechoic collection surrounding a hyperechoic tendon [52]. Thickening of the tendon sheath may also be seen (a normal tendon sheath appears as a thin, circumferential hypoechoic rim measuring less than 1 mm) [52]. In chronic cases, the tendon itself may appear thick and demonstrate abnormal echogenicity (Fig. 10.11). There may be hyperemia of the adjacent bone [52]. A study focusing on MRI evaluation of de Quervain tenosynovitis reported that increased tendon thickness and peritendinous edema are the most reliable findings [53] (Fig. 10.12).
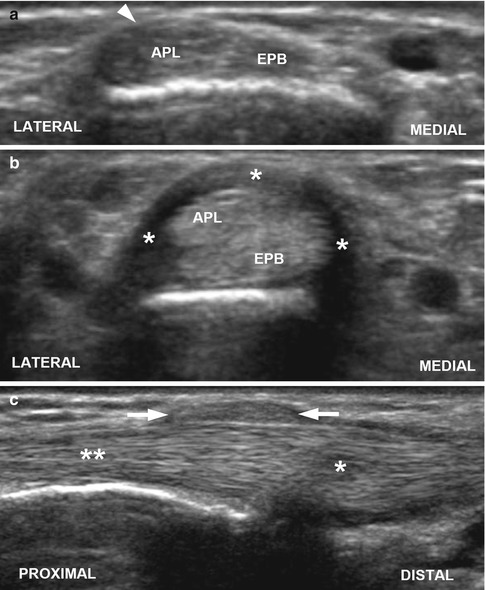
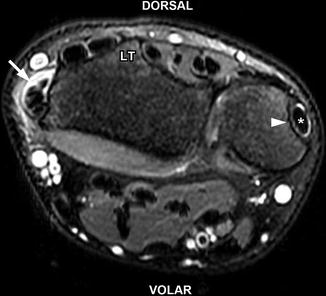

Fig. 10.11
de Quervain tenosynovitis in a 17-year-old girl. (a) Normal side. Transverse US through the first extensor compartment of the wrist demonstrates the normal appearance of the abductor pollicis longus (APL) and extensor pollicis brevis (EPB) tendons with overlying barely perceptible retinaculum (arrowhead). (b) Symptomatic side. Transverse US through the first extensor compartment shows the heterogeneous and thickened APL and EPB tendons along with a thickened extensor retinaculum (asterisks). (c) Longitudinal US of symptomatic side shows heterogeneity and thickening of the distal APL tendon (single asterisk) with thickening of the overlying extensor retinaculum (arrows) consistent with tenosynovitis. Note the more normal appearance of the proximal tendon (double asterisk)

Fig. 10.12
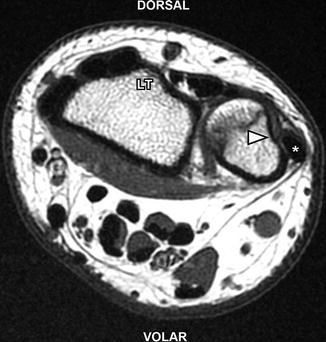
de Quervain tenosynovitis in a 17-year-old girl. Axial T2-W fat-suppressed MRI shows abnormal fluid (arrow) surrounding the abductor pollicis longus and extensor pollicis brevis tendons of the first extensor compartment consistent with tenosynovitis. Mildly increased T2 signal within the tendons indicates tendinosis. There is mild adjacent subcutaneous edema. Note the normal position of the extensor carpi ulnaris (asterisk) tendon within its groove (arrowhead). LT Lister’s tubercle
Differential Diagnosis
Other pathologic findings in the region of the first extensor compartment, such as intersection and Wartenberg syndromes, must be differentiated from de Quervain tenosynovitis.
Sixth Extensor Compartment
Disruption of the extensor carpi ulnaris tendon has been observed in more athletic populations, particularly with racquet sports [54].
Disorders of the extensor carpi ulnaris tendon include tendinosis, tenosynovitis, subluxation, dislocation, or rupture. This tendon lies within the sixth extensor compartment of the wrist and passes through a fibro-osseous sheath formed by the distal ulna and an overlying extensor retinaculum. The anatomical features of this compartment make the tendon less mobile than other extensor tendons, thereby predisposing it to injury particularly during supination and ulnar deviation [54].
Imaging
Tendon subluxation or dislocation is best detected with dynamic ultrasound demonstrating abnormal excursion of the tendon with certain maneuvers. MRI may demonstrate nonspecific synovitis of the extensor carpi ulnaris but cannot demonstrate dynamic instability [55] (Fig. 10.13). The remaining pathologies, including tendinosis, tenosynovitis, and rupture, are clearly demonstrated by both MRI and US [55].