, Valery Kornienko2 and Igor Pronin2
(1)
N.N. Blockhin Russian Cancer Research Center, Moscow, Russia
(2)
N.N. Burdenko National Scientific and Practical Center for Neurosurgery, Moscow, Russia
Before primary tumor cells are transferred to the brain, they grow into the surrounding lymph and blood vessels. Once in the lymph vessels and, rarely, blood vessels, single tumor cells or groups of cells migrate with the current of blood or lymph. A tumor embolus must preserve its viability after it overcomes the action of the immune system or another humoral body defense system, turbulence, and circulation, and only then it “settles” in the capillary bed of the recipient organ, penetrates its parenchyma, proliferates, and forms micrometastases. Not all tumor cells that get into the lymph or blood flow will eventually provide the basis for the growth of a metastatic tumor. Thus, according to Liotta and Kohn (2003), the number of tumor microemboli that eventually form metastases is about 0.01%. The potential to metastasize depends on the number of tumor cell emboli and the specifics of interaction between the latter and homeostatic mechanisms of the host (Fidler 1997; Langley and Fidler 2013).
The development of most cancers is associated with the need for a blood supply sufficient to meet the needs of increased tumor metabolism. This, in turn, leads to the formation of additional, abnormally shaped and branched vasculature, so-called abnormal tumor vasculature (Shweiki et al. 1992; McDonald and Choyke 2003; Pronin et al. 2005; Bulakbasi et al. 2005). According to Li et al. (2000), as early as 24 h after an injection of 20–50 tumor cells to animals, healthy vessels surrounded by the tumor begin to deform and take a convoluted shape. All the above conditions result in the formation of a relatively independent vascular structure of a metastasis, different from the normal intracranial vasculature. Thus, vascular proliferation and tumor angiogenesis are the most important factors in the biology of secondary brain malignancies (Chaudhry et al. 2001; Fitzgerald et al. 2008).
Leenders et al. (2003) pointed out that the process of metastasizing is highly specific and involves some successive transformations. After implantation of the embolus cell and primary cell growth, the tumor tissue should become vascularized (it is usually noticeable when the tumor mass reaches 1 mm3 in diameter). Vascular endothelial growth factor (VEGF) that primarily affects the activity of tumor vasculature formation also affects the surrounding tissue, which results in the formation of anastomoses between the vasculature, the tumor, and the body. This factor can promote initial tumor growth even without “own” tumor angiogenesis (Kusters et al. 2003).
A metastatic tumor growing in the brain parenchyma certainly interacts with various protective mechanisms and structures, particularly, with the structures of the blood-brain barrier (BBB). In contrast to primary lesions, metastatic tumor cells should reach brain microvessels, “attach” to their endothelium, and then penetrate into the brain parenchyma, start active proliferation, and produce a number of factors to promote angiogenesis. Formation of a local metastasis starts with the interaction of tumor cells and BBB endothelial cells, which occurs with active contribution of growth factors (Nicolson et al. 1996; Kim et al. 2004). Inhibition of VEGF and activation of anti-angiogenic factors greatly reduce the chance for a metastatic tumor to “catch” in the brain tissues. It should be noted that certain of these endogenous factors, on the contrary, “help” metastatic cells to grow into the brain matter. Analysis of cases with patients treated with adjuvant chemotherapy showed that administered drugs may affect the BBB, so that the probability of metastasizing into the brain increases (Bouffet et al. 1997; Kolomainen et al. 2002; Bendell et al. 2003).
The main elements of the BBB structure are endothelial cells. Intercellular gaps between endothelial cells, astrocytes, pericytes, and BBB are smaller than the gaps between the cells in other tissues. These three cell types represent the structural basis of the BBB (Fig. 2.1). Cerebral vessels are characterized by the presence of tight junctions between endothelial cells and the absence of both fenestrations and intercellular gaps between them. Tight junctions between endothelial cells inhibit intercellular (paracellular) passive transport. Thus, the endothelial lining of the brain capillaries is continuous. Another difference between the cerebral capillary endothelium and the peripheral capillary endothelium is a low content of pinocytosis vesicles. All of this is aimed at preventing the penetration of various unwanted substances from the bloodstream into the brain tissue.
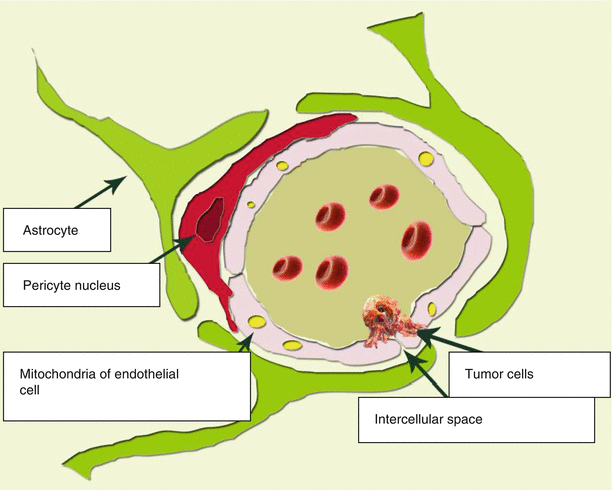

Fig. 2.1
The structural BBB unit. A distinctive characteristics of the relationship between a capillary and the brain substance is a close contact of endothelial cells, as well as presence of pericytes with a contractile function, which prevents large blood elements from penetration outside the capillary
Every second to fourth endothelial cell has a contact with a pericyte. Pericytes are mainly located at the points of contact of endothelial cells. Pericytes are present in almost all arterioles, venules, and capillaries of the body. The level of their coverage of the endothelial capillary layer correlates with the permeability of the vascular wall. Pericytes are tightly bound to endothelial cells. This binding occurs through three types of contacts: gap junctions, focal adhesions, and membrane invagination from one cell to the cavity of another. Gap junctions directly connect cytoplasm of two cells, while being permeable to ions and small molecules. This is typical only for cerebral pericytes. They perform the macrophage function in the cerebral capillary network. Accordingly, the cytoplasm of cerebral pericytes contains a large number of lysosomes. The ability of pericytes to perform phagocytosis and antigen presentation was confirmed in tissue culture (Peppiatt et al. 2006).
Pericytes also synthesize a number of vasoactive substances and play an important role in angiogenesis. Macrophage properties of pericytes constitute a “second line of defense of the brain” from neurotoxic molecules that have overcome the barrier of endothelial cells. Thus, they are an important part of the cerebra immune system, which, along with other factors, significantly hinders penetration of metastatic cells into the brain substance.
In particular, the above properties of neoplastic growth of colorectal cancer are controlled by the genes responsible for angiogenesis, invasion, and metastasizing that promote production of so-called matrix metalloproteinases (MMPs) by tumor cells (Delektorskaya et al. 2007; Ganusevich 2010; Said et al. 2014). The MMP1, MMP2, and MMP9 expression was demonstrated to represent an unfavorable prognostic factor for colon cancer . Similar associations were found for other protease family—uPA (urokinase-type plasminogen activator). Glycoprotein CD44 performing the adhesive function and apparently promoting the attachment of tumor cells in anatomically distant tissues and organs is one of the best known markers of metastasizing (Pasche et al. 2002; Kahlenberg et al. 2003).
In case of invasion of melanoma cells, for example, neurotrophins—proteins that support the viability of neurons and stimulate their growth and activity—facilitate local destruction of the basement membrane of the BBB cells and promote the release of angiogenic factors by increasing the production of extracellular matrix (ECM) degradation enzymes, such as heparanase. Heparanase produces an effect on the growth of melanoma and other malignant tumors and promotes their invasion into distant organs. There is an increased number of neurotrophins on the tumor-brain interface (Nakajima et al. 1988; Yano et al. 2000; Vlodavsky and Friedmann 2001; Denkins et al. 2004).
Findings of Wikman et al. (2012), who studied the profiles of chromosomal aberrations (whole genome) in primary breast cancer and brain metastases using comparative genomic hybridization (CGH) to microarrays, showed that brain metastases in general contain the same chromosomal aberrations as the primary tumor, but their occurrence is a few times higher. A statistically significant difference was obtained for nine different loci with an increased EGFR (7p11,2) content and amplification—epidermal growth factor receptor from the tyrosine kinase group—and a decrease in 10q22,3-qter as the most relevant and significant aberrations in the metastasis (p < 0.01, false positives <0.04). An allelic mismatch in 10q was confirmed in 77 primary tumors and 21 metastases. A mismatch in the PTEN locus (phosphatase and tensin homolog), an enzyme that suppresses the tumor cell activity, was greater in metastases (52%) and in primary tumors with a brain relapse (59%) as compared to primary tumors (18%, p = 0.003) or a non-brain relapse (12%, p = 0.006). A decrease in PTEN expression is most common in HER-2 negative metastases (64%). Furthermore, expression of a micro-PHK PTEN was decreased in brain metastases as compared to the primary tumor. PTEN mutation was often detected in metastases. These findings demonstrate that brain metastases contain a very complex set of genomic aberrations, suggestive of a possible role of PTEN and EGFR in their formation.
The ability of invasive growth and metastasis growth rate are associated with its original nature. This theory is based, according to Radinsky et al. (1998), on the following three principles. First, tumors are biologically heterogeneous and contain subpopulations of cells with a variety of angiogenic, invasive, and metastatic properties. Second, metastatic process depends on the activity of invasion, embolization, survival of the tumor embolus cell in the circulation, and its entrapment in distant capillaries and spread with cell division in the parenchyma of the target organ. Third, a great deal depends on the multiplicity of invading cells, their ability to oppose the homeostatic mechanisms of the host. The tumor can “attract” inflammatory cells that provide more favorable conditions for a further growth of metastases and “affect” the BBB (Egeblad and Werb 2002). During the development of a metastatic tumor in the brain, its volume increases, and it invades into the brain tissue underdriven by growth factors produced by astrocytes (provided the tumor cell has specific receptors) (Gavrilovic and Posner 2005).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree