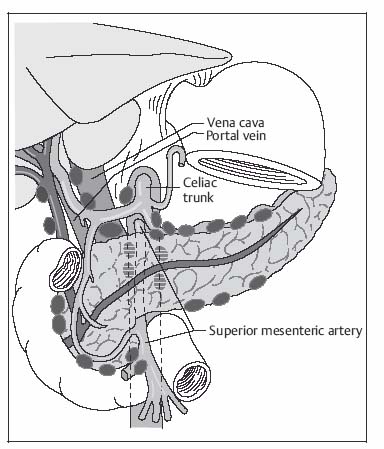
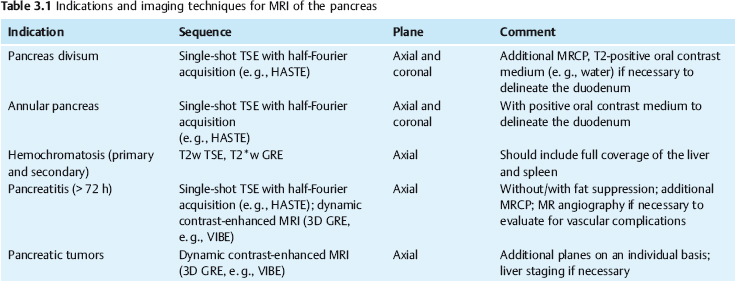
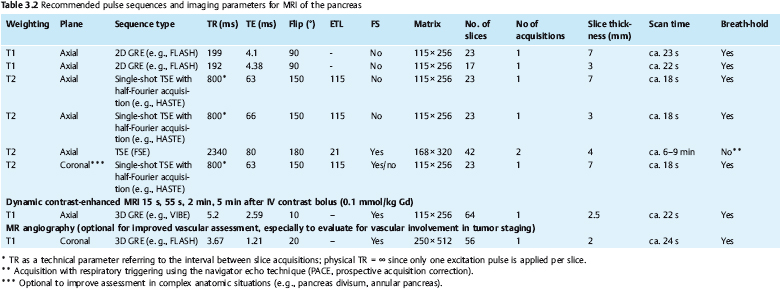
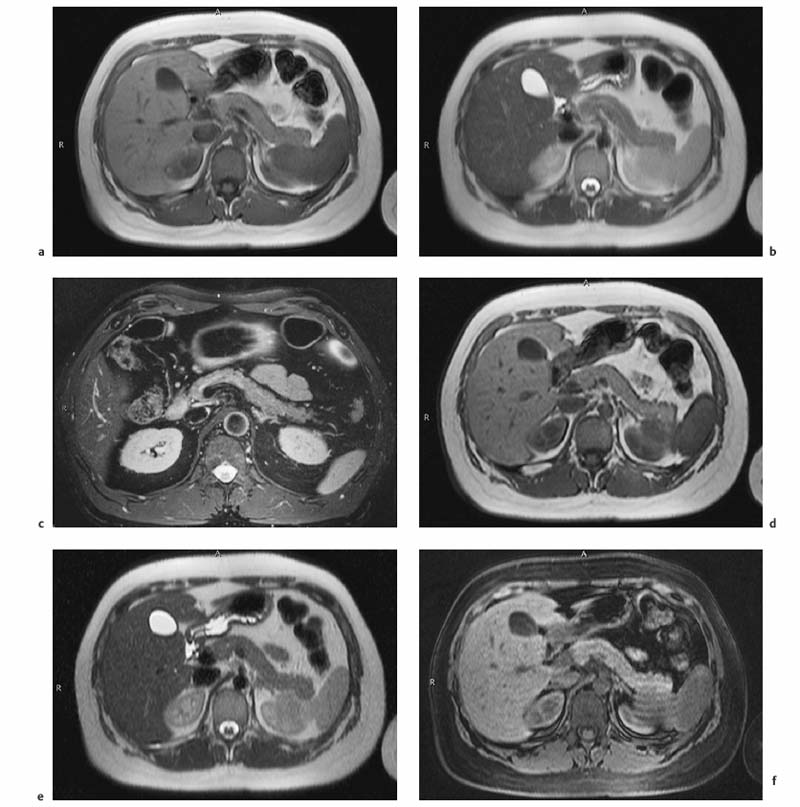
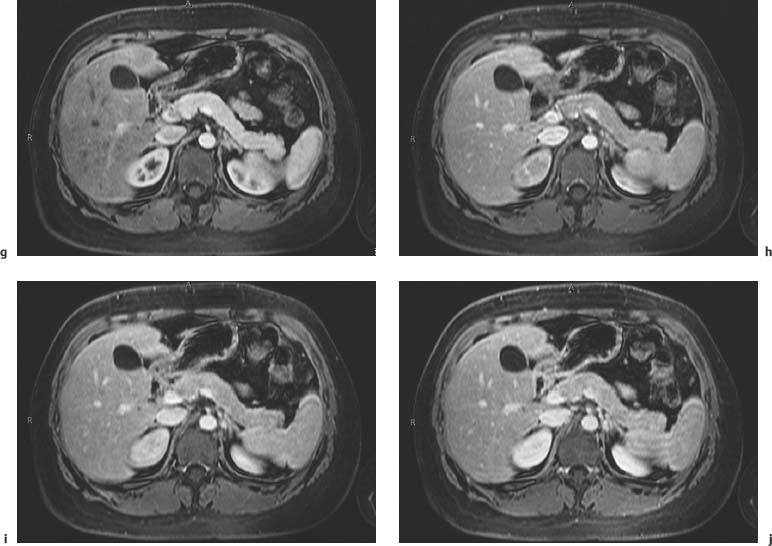
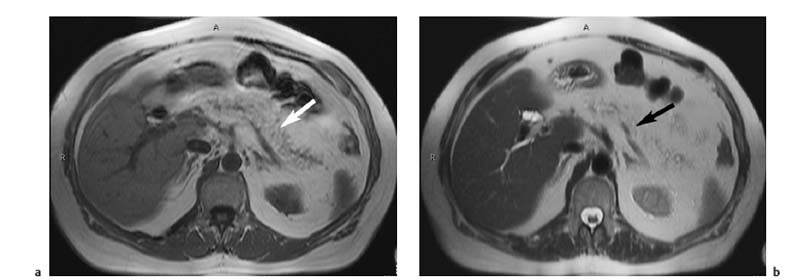
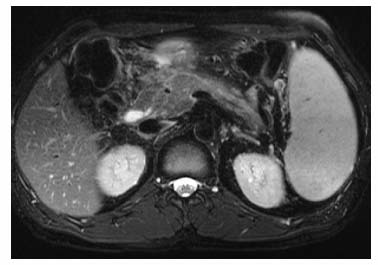
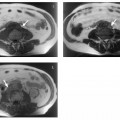
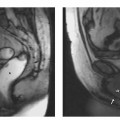
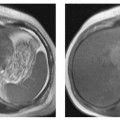
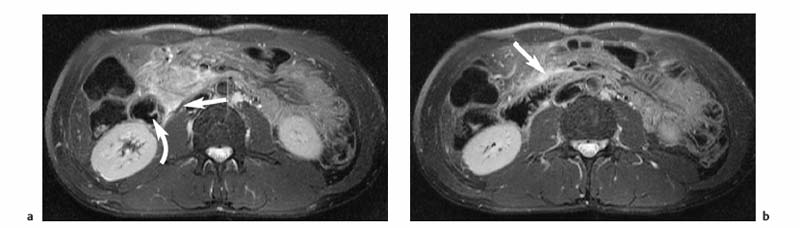
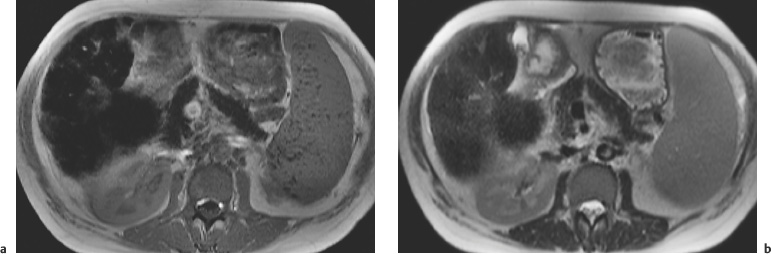
3 The Pancreas The pancreas has two very different functions in the regulation of metabolism, which is why functional disorders present with a variety of clinical symptoms. Treatment is complex and may include pancreas transplant as a final option in some patients. Insofar as specialized cells (grouped in the islets of Langerhans) secrete several important hormones—insulin, glucagon, somatostatin, and pancreatic polypeptide—the pancreas is an endocrine organ. It is also an exocrine organ, producing several purely serous secretions with important digestive functions in the gastrointestinal tract: proteases (trypsin, chymotrypsin, and others), esterases (including lipase), carbohydrases, and nucleases. The pancreas is therefore made up of glandular tissue and a system of ducts. It is embedded between the liver, stomach, and duodenum (Fig. 3.1) and is a highly vascularized organ with multiple blood supply. Since the pancreas lacks a capsule and is in close vicinity to the vessels and organs of the upper abdomen, pancreatic disease often involves these structures. The clinical presentation is chronic to hyperacute. The high intrinsic soft-tissue contrast justifies the use of MRI as the primary imaging modality in the diagnosis and staging of pancreatic tumors (including identification of nodal and distant metastases) and evaluation of patients for normal anatomic variants (Table 3.1). MRI using fast breath-hold sequences with high spatial resolution has been shown to be superior to CT and ultrasound in the detection of pancreatic lesions.1–3 Moreover, MRI offers a wider range of diagnostic options because it enables non-invasive 3D imaging of the pancreatic duct system using MR cholangiopancreatography (MRCP; see Chapter 2) in the same session. By contrast, initial results suggest that multiplanar reconstruction of multislice spiral CT data does not greatly increase the sensitivity of CT in detecting pancreatic tumors.4 However, results are expected to improve given the ongoing rapid development of multislice spiral CT technology. CT is currently the preferred imaging modality in the acute setting (acute pancreatitis, trauma) because it is faster and more readily available than MRI. One of the most challenging tasks for radiologists is to differentiate pancreatic malignancy from benign lesions, in particular chronic inflammatory changes with a mass effect. The distinction can be made with a high degree of accuracy by using a combination of MR strategies (axial imaging, MRCP, MR angiography).5 In patients with chronic pancreatitis, MRI is useful in assessing and following up the extent of the disease process and possible effects on adjacent structures (splenic vein thrombosis, perforation of pseudocysts into neighboring organs). In contrast, CT is still superior in identifying pancreatic calcifications and abscess-related air inclusions. As open MR scanners are becoming more widely available, the option of performing interventional procedures (biopsy, drainage, celiac plexus block) under real-time multiplanar MRI guidance is also expected to expand the therapeutic applications of pancreatic MRI. High spatial resolution and good soft-tissue contrast are essential when imaging the pancreas. Therefore, a high signal-to-noise ratio (SNR) is needed. This can be achieved by imaging at high field strength (at least 1 T) an d using a body or torso phased-array surface coil.6 Fat saturation techniques are also more effective at higher field strengths. Motion artifacts can be minimized by using fast breath-hold sequences, which require high-performance gradients, or by using respiratory triggering.7 Respiratory-triggered sequences take much longer to acquire but improve spatial resolution and are therefore an integral part of pancreatic MRI. Application of presaturation bands above and below the field of view (FOV) is recommended to reduce artifacts from inflowing blood. Axial images through the pancreas are usually sufficient to establish a diagnosis. An additional coronal or sagittal sequence may help delineate the pancreatic head from the duodenum and predict the resectability of pancreatic cancer. An MRI protocol for the pancreas is presented in Table 3.2. We start by obtaining a three-plane localizer of the upper abdomen using a breath-hold GRE sequence. Next, fast axial multislice breath-hold sequences (e. g., T1w FLASH, T2w HASTE; Fig. 3.2a, b) are acquired of the pancreas, the entire liver, the spleen, and the upper kidney poles (each within one breath-hold, acquisition time < 23 s). Alternatively, T2w images can be obtained without breath-holding using a respiratory-triggered TSE sequence (e. g., PACE)7 (Fig. 3.2c). The non-breath-hold sequence has higher spatial resolution but is more susceptible to peristaltic artifacts because of the longer acquisition time. Since the high signal intensity of fat improves the conspicuity of the pancreatic contours (Fig. 3.2b), a fat-suppression technique is not applied unless peripancreatic fluid collections are suspected (Fig. 3.2c). Additional axial (coronal slices if necessary) T1w and T2w thin-slice sequences (3 mm) should be acquired for evaluation of ductal structures and identification of very small parenchymal lesions (Fig. 3.2 d, e). A dynamic MR examination after intravenous bolus injection of Gd-based contrast medium (e. g., Omniscan, Magnevist) is recommended for more detailed morphologic evaluation of the pancreatic parenchyma. Arterial phase images obtained 15 s after the contrast bolus (at a standard dose of 0.1 mmol/kg body weight Gd) are most useful. Additional images should be acquired at 55 s, 2 min, and 5 min. The dynamic study is best performed using a thin-slice T1w 3D GRE sequence with high spatial resolution, such as a VIBE sequence (volume-interpolated breath-hold examination) (Fig. 3.2 f–j). Postcontrast images obtained with this GRE sequence at the above times arewell suited for detecting pancreatic lesions6,8,9 and can replace imaging with conventional T1w sequences, which are inferior in terms of spatial resolution and slice thickness. Since MR cholangiopancreatography (MRCP; see Chapter 2) is fast to perform and often provides useful additional information, it should be done routinely in all patients undergoing MRI of the pancreas. Dynamic contrast-enhanced MRI can be dispensed with in favor of MR angiography to rule out splenic vein thrombosis or vascular infiltration in patients with a pancreatic tumor or severe pancreatitis. Dynamic contrast-enhanced MRI for evaluating perfusion of the pancreas at different times can be performed after intravenous injection of a nonspecific, Gd-based contrast medium (e. g., Magnevist, Omniscan) at a dose of 0.1 mmol/kg body weight (see above). As with CT,10 arterial phase images (15 s after the contrast bolus) are most suitable for parenchymal evaluation and tumor detection.8 Determination of individual circulation time after administering a test bolus (2 mL) may be advisable to optimize timing of the scan delay for arterial phase imaging in patients with reduced cardiac output. Since paramagnetic contrast media are low-molecular-weight substances with rapid distribution in the extracellular space, focal pancreatic lesions may be obscured on equilibrium phase images.11 Current knowledge suggests that Gd-based contrast media are not likely to adversely affect the course of acute pancreatitis and can therefore be administered at the standard dose.12 Fig. 3.2a–j Illustration of normal findings obtained with standard protocol for MRI of the pancreas (see Table 3.2). a T1w 2D multislice FLASH sequence. b T2w multislice HASTE sequence. c Axial fatsaturated T2w multislice TSE sequence (acquired with respiratory navigator, PACE). d Thin-slice (3-mm) T1w 2D multislice FLASH sequence. e Thin-slice (3-mm) T2w multislice HASTE sequence. Fig. 3.2a–j f–j T1w 3D GRE sequences (VIBE) before (f) and 15 s (g), 55 s (h), 2 min (i), and 5 min (j) after IV contrast bolus (0.1 mmol/kg Gd). Timing of postcontrast image acquisition is less critical when tissue-specific contrast media are used. A tissue-specific contrast medium approved for use in MRI of the pancreas is mangafodipir trisodium (MnDPDP; Teslascan, GE Healthcare). It is administered as a short intravenous infusion, and images are acquired with a delay of ca. 15 min. MnDPDP was originally developed for liver imaging but can also be used to detect focal pancreatic lesions as it markedly enhances the signal intensity of normal pancreas on T1w GRE images.13 Oral contrast media14 are helpful to differentiate the pancreatic head from the duodenum or free fluid from fluid in the intestine (Fig. 3.3). Tap water acts as a positive contrast medium on T2w sequences and is sufficient to improve delineation of the pancreas from the duodenum. A negative oral contrast medium, which reduces or eliminates the signal from the bowel lumen on T2w images, is needed to distinguish between free and endoluminal fluids. Negative contrast media include blueberry juice, which is rich in manganese,15 and iron oxide particles.16 Use of negative oral contrast medium is recommended especially when additional MRCP is performed (see Chapter 2). One must be aware that T1-positive oral contrast media, much like fat, exacerbate motion artifacts and should only be used in conjunction with breath-hold sequences. Oral contrast media on the basis of superparamagnetic iron oxide particles17 will eliminate this problem but may lead to susceptibility artifacts on GRE images if the concentration is too high or agglutination occurs.18 The oral contrast solution (300–600 mL) should be ingested over a period of 30 min before the examination. On the scanner table, the patient can be briefly turned into the left lateral position to improve contrast filling of the duodenum. Peristaltic artifacts can be reduced by intramuscular injection of a spasmolytic. Fig. 3.3a, b Chronic pancreatitis with an acute episode 2 weeks earlier in a 41-year-old man. MRI with negative oral contrast medium. T2w multislice TSE sequence (acquired with respiratory navigator, PACE). The duodenal content has low SI (curved arrow) (a descending part, b horizontal part), while the free peripancreatic fluid (straight arrows) is seen as a thin rim of high SI around the head of the pancreas (a) and immediately below the head (b). The pancreas is located in the retroperitoneum at the level of the first and second lumbar vertebrae. It consists of a head with the uncinate process (which hooks behind the superior mesenteric artery), body, and tail. The head is supplied by the gastroduodenal artery (arising from the common hepatic artery) and the superior mesenteric artery and its pancreaticoduodenal branches. The body and tail are supplied by the pancreatic branches of the splenic artery. The veins draining the pancreas empty into the splenic vein or directly into the portal vein. The most important lymph node stations are located in the porta hepatis, along the celiac trunk, and at the mesenteric root (Fig. 3.1). Most of the pancreatic head is surrounded by the duodenum, the so-called duodenal C-loop. Posterior to the head run the inferior vena cava and the right renal artery and vein with the right transverse colon and gastroduodenal artery lying anterior to it. Posterior to the body of the pancreas are the aorta and the celiac and superior mesenteric arteries arising from it, the superior mesenteric and splenic veins, the left renal vessels, the hilum of the left kidney, and the left adrenal gland; anterior to the body lie the transverse mesocolon and the omental bursa with the posterior gastric wall. The normal pancreatic parenchyma is hyperintense to liver on fat-suppressed T1w SE sequences and isointense on T1w GRE sequences (Fig. 3.2a). It shows a marked and early signal increase on contrast-enhanced arterial phase images, which rapidly returns to almost precontrast intensities (Fig. 3.2 g–j). Partial or complete fatty replacement (lipomatosis) of the pancreas is common in older persons and is characterized by prominent lobulation and a decrease in anteroposterior diameter (Fig. 3.4). Fig. 3.5a, b Primary hemochromatosis in a 34-year-old woman. a T1w 2D multislice FLASH sequence. b T2w multislice HASTE sequence. There is marked hypointensity of the parenchyma of the liver and pancreas on the T2w image. The MR signal reduction caused by iron deposits in affected organs is best appreciated on T2*w images. In primary (congenital) hemochromatosis, iron does not accumulate in the spleen but in the pancreas, which is spared in secondary (acquired) hemochromatosis. Pancreas Divisum Pancreas divisum results when the dorsal and ventral pancreatic anlagen fail to fuse and there is persistence of two separate ductal systems that do not communicate.19 In affected individuals, the secretions produced in the body and tail of the pancreas drain through the accessory pancreatic duct (duct of Santorini), which courses in the anterior part of the pancreatic head and enters the duodenum through the minor papilla. Pancreas divisum has a reported prevalence of 1.5–10%.19–21 Although pancreas divisum is a normal anatomic variant, affected individuals may suffer from recurrent episodes of pancreatitis due to functional stenosis obstructing the outflow of exocrine juices through the minor papilla.22,23 Improvement can be achieved by sphincteroplasty, sphincterotomy, or stenting.24 In approximately 42 % of patients with pancreas divisum, the pancreatic head is enlarged and can sometimes mimic a tumor.25 MRCP may be helpful in diagnosing pancreas divisum (see Chapter 2, Fig. 2.5). Annular Pancreas Annular pancreas is an extremely uncommon congenital anomaly characterized by a ring of normal pancreatic tissue encircling and obstructing the descending duodenum. To diagnose this condition, administering oral contrast medium and acquiring coronal images to supplement the standard axial sequence is recommended.26 Pancreatic Cysts Pancreatic cysts (which are true cysts and must not be confused with pseudocysts, see below) typically occur in association with cysts in the liver, kidneys, or cerebellum and in patients with von Hippel–Lindau disease. They are clearly identified by their high signal intensity on T2w images and, unlike cystic tumors, do not enhance after contrast administration. Cystic Fibrosis Cystic fibrosis (mucoviscidosis) is an autosomal recessive disease causing fibrosis in the pancreas and lungs. Three distinct patterns of pancreatic involvement have been noted27: All three patterns can be distinguished by MRI. The degree of fatty replacement can be estimated by comparing T1w images acquired without and with fat saturation. Primary Hemochromatosis Primary (hereditary, idiopathic) hemochromatosis is an autosomal recessive disorder of iron storage. Excessive absorption of ingested iron in the jejunum and a limited capacity of the mononuclear phagocyte system (MPS) to cope with the excess iron lead to deposition of hemosiderin in tissue, particularly in the liver, heart, gonads, skin, and pancreas (Fig. 3.5). The iron overload causes the islet cells to atrophy and can ultimately result in endocrine dysfunction (clinically manifesting as bronze diabetes). Stored iron has higher susceptibility (i. e., is more readily magnetized) and thus distorts the magnetic field, shortening T2 and T2* relaxation times and reducing signal intensity on T2w and T2*w images (Fig. 3.5b). The signal reduction is best appreciated on T2*w GRE images. SE sequences are less sensitive in detecting iron because stored iron interacts directly with the nuclear spins and magnetic field inhomogeneities are compensated for by the 180° refocusing pulse. Secondary Hemochromatosis In secondary (acquired) hemochromatosis (also known as hemosiderosis), the iron overload is due to increased erythrocyte breakdown and accumulation in the phagocytosing system (e. g., Kupffer cells in the liver). Causes may be internal (ineffective or hypoplastic erythropoiesis) or external (increased iron intake, e. g., through repeat transfusion). The pancreas is usually spared in secondary hemochromatosis and retains its normal signal intensity, while the liver shows the same iron-related signal changes as in primary hemochromatosis.28 Acute Pancreatitis Acute pancreatitis is a common condition with an incidence of 5–10 cases per 100 000 population and a peak between 40 and 60 years. The most common causes are cholelithiasis (60–70 %) and alcohol abuse (20–30 %). Less common causes include autoimmune diseases, genetic defects,29
P. Asbach, W. Luboldt, and H.B. Gehl
Introduction
Indications
Imaging Technique
Imaging Planes
Pulse Sequences
Contrast Media
MRI Appearance of Normal Anatomy
MRI Appearance of Pathologic Entities
Congenital Anomalies and Diseases
Inflammatory Disease
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree