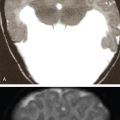
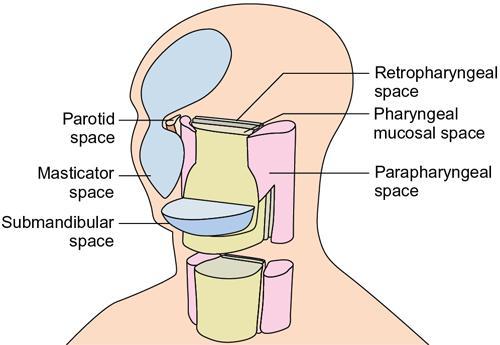
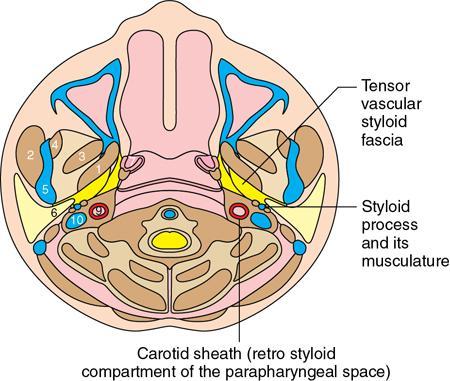
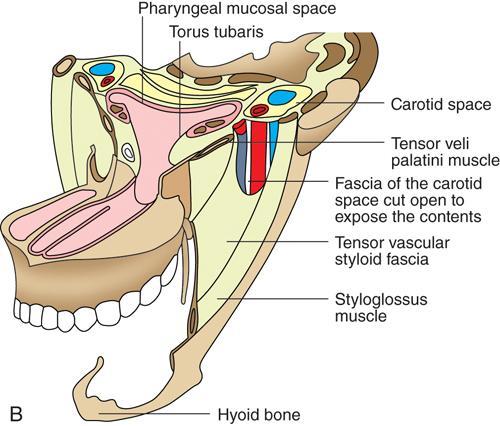
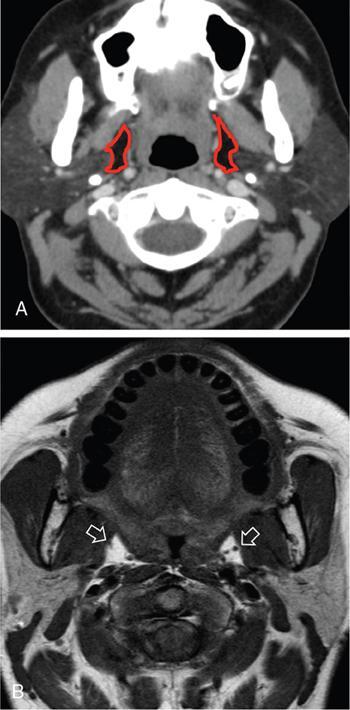
Praveen Kumar Chinniah, Geethu Elizabeth Punnen, Anish Krishna, Soumya Susan Regi The parapharyngeal space, an inverted pyramidal structure, extending from the skull base to the hyoid bone, forms the anatomic centre of the suprahyoid neck, being surrounded by the four important key spaces including pharyngeal mucosal space, masticator space, retropharyngeal space and the parotid space. Being a deep-seated space, clinical examination as well as sonographic evaluation of pathologies involving this space plays a limited role. Computed tomography (CT) and Magnetic Resonance imaging (MRI) form the key modalities in the evaluation of these pathologies, with the aim of differentiating true lesions arising from the parapharyngeal space from pathologies from surrounding spaces extending to the parapharyngeal space, as the differentials for these vary. As the name implies, the parapharyngeal space is located on either side of the nasopharynx and the oropharynx, extending from the skull base to the level of the hyoid bone resembling a hatchet (Fig. 3.13.1). Encircled by other deep neck spaces, parapharyngeal space can be considered the epicenter of the supra hyoid neck. The parapharyngeal space is divided into an anterolateral prestyloid compartment and posteromedial poststyloid component by a fascia called the tensor–vascular–styloid fascia. This fascia covers the styloid process and its musculature, runs anteromedially to join the fascia of tensor velipalatini muscle and finally merges with the pterygomandibular raphe and the buccopharyngeal fascia (Fig. 3.13.2). Division of the parapharyngeal space into prestyloid and poststyloid components has been a bone of contention amongst anatomists, surgeons and radiologists. Anatomists and surgeons consider the poststyloid compartment to be an integral part of the parapharyngeal space. The poststyloid compartment is essentially the carotid sheath and its contents traversing from the infrahyoid neck to the skull base. Hence, radiologists consider the poststyloid compartment as the carotid space and prefer to the prestyloid compartment as the parapharyngeal space. In this chapter, the prestyloid compartment has been regarded as the ‘true’ parapharyngeal space (PPS) and dealt with in detail. Some of the major differences between the prestyloid/true parapharyngeal space and the poststyloid/carotid space have been listed in Table 3.13.1. The carotid space is separately discussed in the Chapter on Carotid Space. Simulating an inverted pyramid, the PPS has a base, anterior border, medial border, posterior border, lateral border and an apex (Table 3.13.2). Inferior surface of the temporal bone forms the base of PPS. Anteriorly, the PPS is limited by the pterygomandibular raphe and posteriorly by the carotid sheath (Fig. 3.13.3A). PPS is sandwiched between a muscle of deglutition and a muscle of mastication on medial and lateral aspects, respectively. The buccopharyngeal fascia that covers the outer aspect of superior constrictor of the pharynx forms the medial boundary. The anterolateral boundary is formed by the superficial layer of the deep cervical fascia covering the medial border of the medial pterygoid. Posterolaterally, the PPS is closely related to the parotid space. Inferiorly, the PPS is considered to extend till the level of hyoid bone. However, the confluence of multiple fascial reflections (from the structures adjoining the superior border of the deep lobe of the submandibular gland) seals the PPS at a cranial level. This premature closure eventually makes the styloglossus as the functional inferior limit of PPS like an apex (Fig. 3.13.3B). Antero-inferiorly, the PPS communicates with the submandibular space acting as a potential route of disease spread. PPS is predominantly filled with fat. The other contents include the ascending pharyngeal and the internal maxillary arteries, pterygoid venous plexus, branches of mandibular division of trigeminal nerve (V3), cervical sympathetic trunk and minor salivary glands. The deep lobe of the parotid gland projects into the PPS through the space between the posterior border of mandibular ramus and the styloid process, called the ‘stylomandibular tunnel’. Due to its rich fat content, the PPS can be readily recognized on both CT and MR imaging (Fig. 3.13.4A and B). Displacement or obliteration of the fat in the PPS greatly aids in localizing the spatial origin of the suprahyoid neck lesions. Parapharyngeal masses remain undetected for a long time, as they do not cause symptoms unless they grow to 2.5–3 cm in size. They are often discovered as an incidental finding when patients are examined or undergo imaging for an unrelated problem. The most common presentation is as a painless neck mass or an intraoral bulge or mass. As masses of the parapharyngeal space enlarge, they displace the most pliable lateral pharyngeal wall medially which may cause the sensation of a ‘lump in the throat’. Other less common symptoms reported are otalgia, dysphagia, facial pain and parasthesias, dysphonia, sore throat, hearing loss, tinnitus and obstructive sleep apnea. Patients with infection involving the parapharyngeal space present with fever, trismus, odynophagia, perimandibular oedema. Lower cranial neuropathies manifest when the carotid space is involved, especially with malignant tumours when patients present with difficulty in swallowing or with hoarseness of voice secondary to vocal cord paralysis. Other features that raise concern for a malignant pathology include history of rapid growth, presence of a mass extending outside of parapharyngeal space or a mass associated with severe pain. Lower cranial nerve neuropathies do not necessarily point towards a malignant pathology. An infected parapharyngeal second branchial cleft cyst or large neurogenic tumours may present with neurological symptoms involving the lower cranial nerves or sympathetic chain. As the parapharyngeal space lies deep to the mandibular ramus, pterygoid muscles and the parotid gland and immediately lateral to the pharynx, bimanual palpation is a useful examination technique to detect smaller masses (1–1.5 cm). Larger masses (1.5–4 cm) on intraoral examination or flexible endoscopy demonstrate pharyngeal fullness or bulge. With further increase in size, the mass can fill the entire parapharyngeal space, displacing the parotid laterally with resultant fullness near the angle of mandible. Other palpatory clues such as compressibility, pulsatile nature or presence of a thrill may suggest the possibility of a vascular tumour. The deep-seated location of the parapharyngeal space within the neck limits clinical evaluation. Thus, clinicians significantly rely on radiological imaging to assess not only the site of origin, characteristics, extent and probable nature of the mass, but also its vascularity and relationship to the great vessels of the neck and other neurovascular structures, particularly as surgery forms the mainstay of treatment of these lesions. The goals of imaging include the following: The deep-seated nature of this space and overlying mandible, maxilla, styloid and mastoid processes limit the role of ultrasonography in the evaluation of parapharyngeal space pathologies. They may be useful in large tumours, which present more superficially in the neck. Intraoral ultrasound may guide needle biopsy or aspiration if intraoral route is being considered. CT and/or MRI are the imaging modalities of choice to evaluate lesions of the parapharyngeal space. The fat within the parapharyngeal space is readily delineated on both CT and MRI (Fig. 3.13.4A and B). CT is particularly useful for tumour calcification and bony erosion. Imaging is acquired as axial sections from skull base to the upper border of manubrium sternum, preferably with contrast; 3–5 mm thickness contiguous slices, with a small field of view (FOV). Coronal and sagittal reconstructions may then be reformatted from the acquired axial sections. Additionally, high-resolution bone algorithm accurately evaluates bone involvement if any. Though CT has the advantages of being readily accessible, is faster, hence requiring less patient cooperation and suitable for patients with contraindications to MRI such as implanted pacemakers or cochlear implants, it generally provides less information as compared to gadolinium-enhanced multiplanar MRI imaging of this region and also utilizes ionizing radiation. MRI is superior in defining soft tissue abnormalities and providing important information on malignant signs such as local invasion, regional metastasis and perineural invasion. As fat is a prominent element in the parapharyngeal space, and helps in evaluation of lesions in this area, precontrast T1W images should always be obtained without fat suppression. Vascular flow voids are also more reliably interpreted on MRI. A combination of CT and MRI allows for a more complete evaluation of the neurovascular structures, glandular tissue and its relation to the skull base (Table 3.13.3). Imaging plays a significant role in the preoperative diagnosis of parapharyngeal space lesions. Preoperative imaging analysis of the PPS is performed with the aim of providing the following information: Imaging is also used to guide biopsy of some of the lesions in the preoperative evaluation of these lesions. Routine biopsy of palpable PPS lesions that bulge into the pharyngeal mucosa may be performed by means of a peroraltransmucosal approach. However, this ‘blind’ approach poses significant risk of inadvertent injury to the carotid vessels, jugular vein and facial nerve, which may be adjacent to the area of interest. The use of intraoral ultrasound may help in safely guiding the needle into any mass visualized by intraoral ultrasound. For deep-seated head and neck lesions requiring percutaneous biopsy, CT is the imaging modality of choice. Subzygomatic, retromandibular and paramaxillary approaches have been described for safe biopsy of PPS masses. Though MR image guidance can also be used to biopsy PPS lesions, the lack of availability of open configuration MR imaging systems, need for MR compatible needles and higher costs have prevented its widespread use. PET-CT is useful in the evaluation of malignant tumours/nodes, including lymphoma in the PPS. It plays a role in initial staging/diagnosis of an unknown primary as well as treatment assessment and surveillance. The PPS is filled with fat and clearly delineated on CT and MRI (Fig. 3.13.4). Since the PPS has relatively few internal structures, primary lesions of the PPS are rare (Table 3.13.4). The PPS is more commonly displaced or infiltrated by lesions arising in the adjacent spaces. The first step to evaluate any lesion in the PPS is to confirm if the space of origin of the abnormality is truly in the PPS and not secondary extension into the PPS from a bordering space. A mass arising from the parapharyngeal space has a margin of fat separating it from the surrounding spaces (Fig. 3.13.5). The lack of this intervening fat plane suggests that the lesion is not a primary PPS lesion or that it is a PPS lesion, which has infiltrated the adjacent space. Displacement pattern of PPS fat reveals the site of origin of a neck mass. Masses from the spaces surrounding the PPS displace the PPS fat in a predictable manner (Table 3.13.5, Fig. 3.13.6).
3.13: The parapharyngeal space: Prestyloid parapharyngeal space
Introduction
Anatomy of the parapharyngeal space
Introduction
Radiological anatomy
Nomenclature and history
Prestyloid Parapharyngeal Space
Carotid Space (Poststyloid Parapharyngeal Space)
Contents: Fat, minor salivary glands, ascending pharyngeal, maxillary artery, nerves
Contents: Carotid artery, IJV, vagus nerve, IX–XII cranial nerves, sympathetic chain, paraganglia
Extension: Ends at the level of hyoid
Extension: Continues to lower neck
Location: Anterior to styloid and associated muscles
Location: Posterior to styloid and associated muscles
Common pathologies: Minor salivary gland tumours, schwannomas
Common pathologies: Schwannomas, paragangliomas, vascular
Lesions displace carotid artery posteriorly
Lesions displace carotid artery anteriorly
Boundaries
Boundaries
Base
Temporal bone
Anterior
Pterygomandibular raphe
Posterior
Carotid sheath
Medial
Superior constrictor of pharynx
Lateral
Medial pterygoid muscle (anterolaterally) and parotid space (posterolaterally)
Apex
Styloglossus muscle
Contents
Fat, ascending pharyngeal artery, internal maxillary artery, pterygoid venous plexus, branches of mandibular division of trigeminal nerve (V3), cervical sympathetic trunk and minor salivary glands
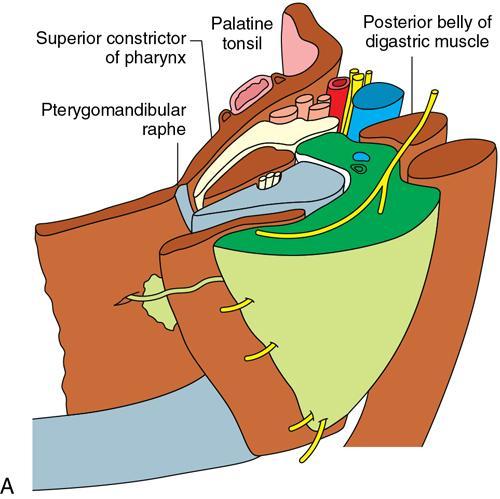
Contents
Clinical features
Role of imaging
Imaging modalities
Ultrasonography
CT and MRI
CT
MRI
Readily accessible, faster, hence requiring less patient cooperation
Less accessibility, relatively expensive, time consuming, susceptible to artefacts
Suitable for patients with contraindications to MRI such as implanted pacemakers or cochlear implants
Not suitable for patients with incompatible implants.
Poor soft tissue resolution, however, allows better evaluation of bony structures
Better soft tissue resolution, provides important information on malignant signs such as local invasion, regional and local metastasis and perineural invasion.
Positron emission tomography (PET-CT) imaging
Approach to pathologies in the parapharyngeal space
Aetiology
Examples
Congenital
Atypical second branchial cleft cyst, lymphatic malformation, venous malformation
Inflammatory
Plunging ranula spreading from submandibular space into PPS
Infection
Spreading from pharyngeal mucosal space (PMS), masticator space (MS), parotid space (PS) or retropharyngeal space (RPS); most commonly peritonsillar abscess from palatine tonsil involves parapharyngeal space
Benign tumours
Lipoma, benign mixed tumour (from minor salivary gland, rest in parapharyngeal space)
Malignant tumours
Spreading from PMS, MS, PS or RPS into PPS; most commonly squamous cell carcinoma spreading from naso- or oropharynx (PMS) into parapharyngeal space
Step 1: Space localization
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree