Fig. 16.1
Embryological development of the dorsal and ventral mesentery and the primitive gut. The dorsal mesentery forms the splenorenal ligament (SRL) and gastrosplenic ligament (GSL), which connects the spleen (Sp) and pancreas to the dorsal abdominal wall (a). Final adult position of the dorsal and ventral mesentery and gut (b). The liver (L) has enlarged and moved to fill the right abdominal cavity. The Stomach (S) is connected to the liver via the gastrohepatic ligament (GHL), which is a part of the ventral mesentery. K kidneys
The peritoneum is separated into the supramesocolic and inframesocolic space, for instance, by the transverse mesocolon. The supramesocolic space is further divided into the left and right supramesocolic space by the falciform ligament. The inframesocolic space is partitioned into the left and right inframesocolic space by the obliquely placed small bowel mesentery, likewise (Fig. 16.2).
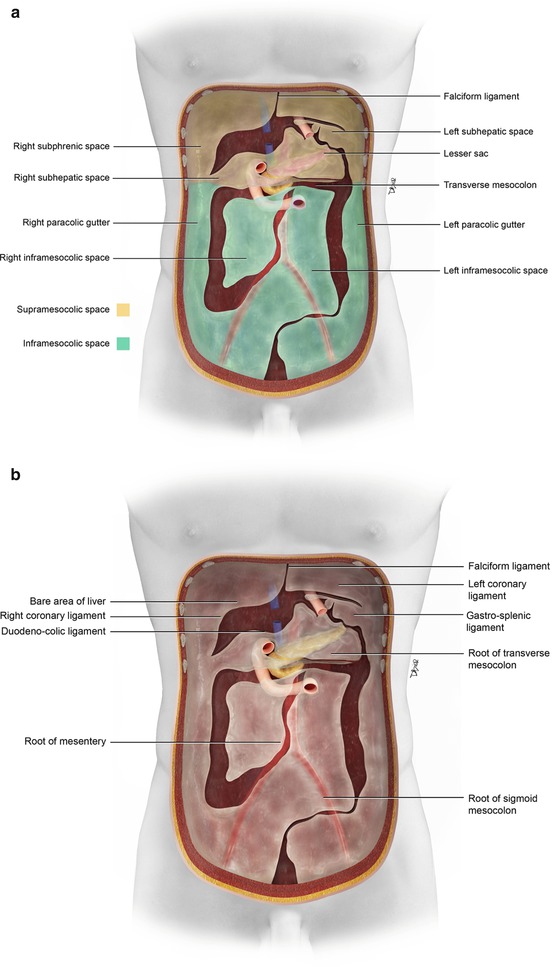
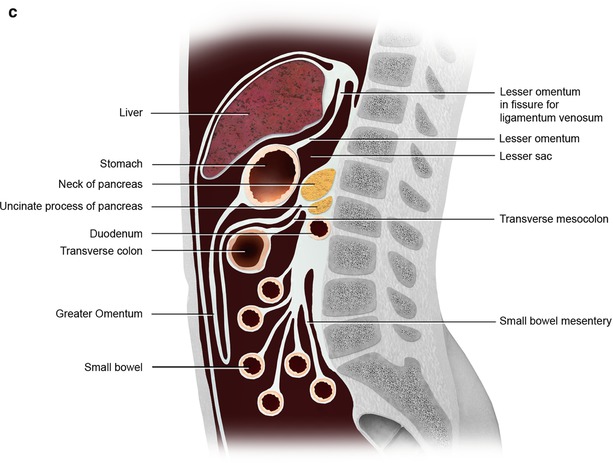


Fig. 16.2
(a) The peritoneum is divided into the supramesocolic and inframesocolic space by the transverse mesocolon. Additional compartments and spaces are formed by the ligaments and mesentery (a). (b, c) The peritoneum and its ligament and mesenteric attachments (b). Sagittal view of the omental, mesenteric, ligamentous, and visceral attachments within the peritoneal cavity (c)
In the pelvis, the peritoneum continues, forming additional spaces and compartments as a result of its folds and reflections. The bladder divides the pelvic peritoneum into the anterior and posterior paravesicular spaces. Further divisions in the pelvic peritoneum are caused by the indentations made by the inferior epigastric artery, obliterated umbilical artery, urachus, uterus, and rectum (Fig. 16.3).
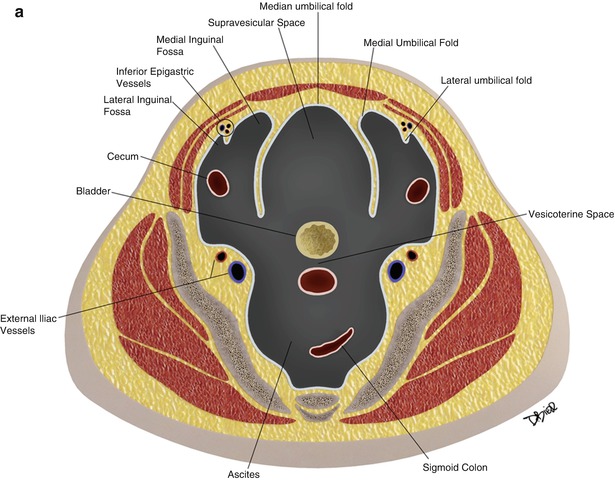
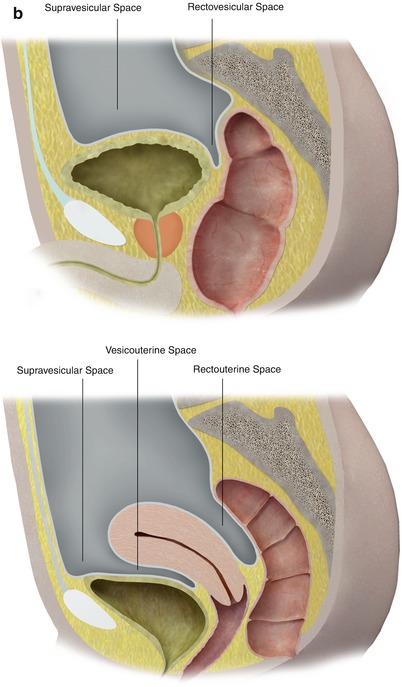


Fig. 16.3
The peritoneum continues into the pelvis, where it is further divided into different compartments and spaces by indentations and reflections of the peritoneum. For example, the lateral and medial inguinal fossae are divided by the indentations made by the inferior epigastric vessels (a). Lateral view of the pelvic peritoneum in the male (A) and female (B) (b)
The complex spaces, compartment, ligaments, mesenteries, and omenta formed from the development of the peritoneum allows for the normal and abnormal pathologic process to occur. It allows for the nourishment of the organs as well as expected routes for disease spread. Disease can spread directly, by intraperitoneal seeding, lymphatic extension and the hematogenous route within the peritoneum. Within this context of development, peritoneal disease manifestations in the form of thickening, masses, cysts, fluids, calcifications, and air can be assessed and understood (Table 16.1).
Table 16.1
Some of the more common pathology of the peritoneum
1. Peritoneal thickening |
(a) Metastasis |
(i) Peritoneal carcinomatosis |
(ii) Mucinous |
(iii) Peritoneal lymphomatosis |
(iv) Peritoneal sarcomatosis |
(b) Primary peritoneal tumors |
(i) Malignant mesothelioma |
(ii) Primary peritoneal serous carcinoma |
(iii) Multicystic mesothelioma |
(iv) Leiomyomatosis peritonealis |
(v) Desmoplastic small round cell tumor |
(c) Infection/inflammation |
(i) Granulomatous peritonitis |
(ii) Sclerosing encapsulating peritonitis |
(d) Miscellaneous tumors and tumor-like lesions |
(i) Endometriosis |
(ii) Splenosis |
(iii) Gliomatosis peritonei |
(iv) Osseous metaplasia |
2. Omental masses |
(a) Acute epiploic appendagitis |
(b) Omental infarction |
(c) Liposarcoma |
(d) Metastasis |
(e) Mesothelioma |
3. Mesenteric masses |
(a) Carcinoid |
(b) Desmoid |
(c) Sclerosing mesenteritis |
(d) Lymphoma |
(e) Metastasis |
4. Peritoneal cystic masses |
(a) Enteric duplication cysts |
(b) Lymphangiomas |
(c) Simple mesenteric or omental cysts |
(d) Female pelvic cysts – peritoneal inclusion cysts/benign cystic mesothelioma, paraovarian cysts, fallopian tube cysts, cystic adenomyosis, and endometriosis |
(e) Nonpancreatic pseudocyst |
(f) Cystic hematomas |
(g) Cystic abscess |
(h) Cystic teratomas |
5. Peritoneal fluid |
(a) Ascites |
(i) Exudate |
(ii) Transudate |
(b) Mucin |
6. Peritoneal calcifications |
(a) Benign |
(i) Chronic bacterial peritonitis, peritoneal tuberculosis, retractile mesenteritis, etc. |
(b) Malignant |
(i) Carcinoid tumor |
(ii) Colon and gastric cancer metastasis |
(iii) Serous tumor of the ovary, etc. |
7. Intraperitoneal air/pneumoperitoneum |
(i) Perforated viscus – peptic ulcer |
(ii) Bowel obstruction |
(iii) Trauma, etc. |
8. Peritoneal/mesenteric defect |
(i) Internal hernias |
9. Peritoneal foreign bodies |
Peritoneal Thickening/Masses
Thickening of the peritoneum is a nonspecific finding. It can be widespread as in the case of peritoneal carcinomatosis or focal. Numerous neoplastic and nonneoplastic conditions cause peritoneal thickening, and their imaging features can overlap. However, several imaging and clinical features can help narrow the differential diagnosis (Algorithm 16.1).
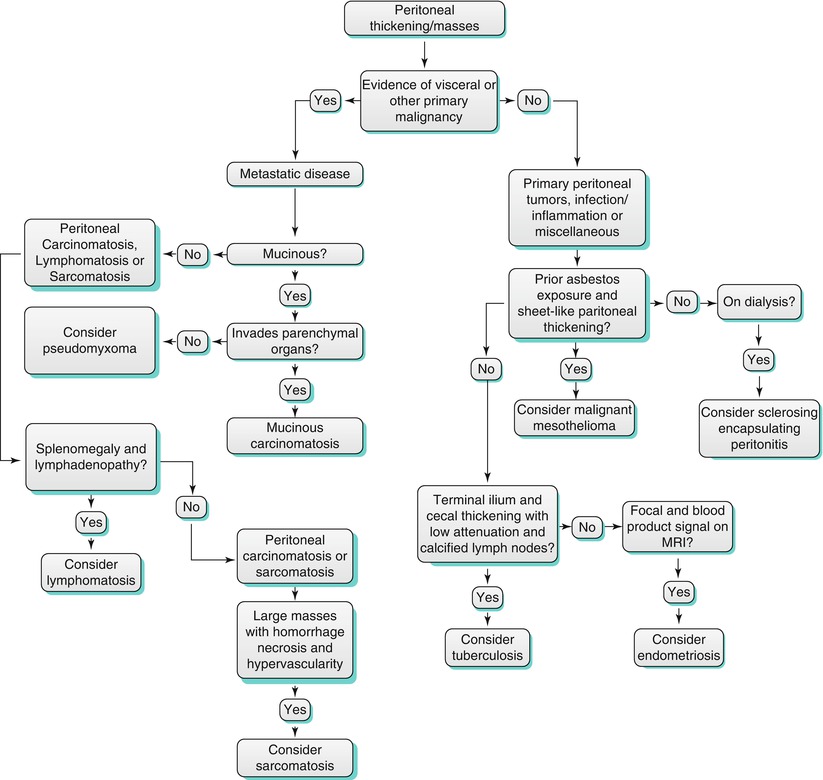

Algorithm 16.1 Flow diagram for the differential diagnosis of peritoneal thickening
For example, if there is evidence of a visceral or primary tumor elsewhere, then metastatic disease would be a concern. Indeed, metastatic disease is the most common cause of peritoneal thickening [2].
If there is no evidence of a primary tumor, then primary peritoneal tumors could be considered. Primary peritoneal tumors such as malignant mesothelioma and primary peritoneal serous carcinoma can cause peritoneal thickening and masses.
In addition, when there is clinical concern for an infectious or inflammatory process, then granulomatous peritonitis from tuberculosis or sclerosing encapsulating peritonitis can be sought.
Finally, when there are focal mass or masses in the peritoneum without a primary malignancy; entities such as splenosis and endometriosis can be present.
Peritoneal Thickening/Metastasis/Peritoneal Carcinomatosis
Again, when there is known ovarian, gastrointestinal tract (stomach, colon, appendix, gallbladder, and pancreas), or other primary malignancies (breast, lung, and uterus), then metastatic disease causing peritoneal thickening should be considered. Metastatic disease to the peritoneum can present as diffuse spread of disease along the extensive surface area of the peritoneum [3]. The ligaments, mesenteries, and compartments formed from the folding of the peritoneum along with the normal circulation of peritoneal fluid dictate the spread of disease [2].
Peritoneal carcinomatosis is defined as widespread metastatic disease in the peritoneal cavity (Fig. 16.4). It is commonly seen as small tumor nodules studding the peritoneal surface, which can later grow and invade the subperitoneal tissues. Omental caking results when tumor nodules have invaded the omental fat and there is an associated fibrotic response (Fig. 16.5). Furthermore, metastatic disease to the ovaries (Krukenberg tumors) forms from the transperitoneal spread of disease. When there is involvement of the mesentery, a sheetlike growth pattern can be seen, similar to malignant mesothelioma. Ascites can be present with peritoneal carcinomatosis. A known complication of peritoneal metastatic disease is small bowel obstruction (Fig. 16.6).
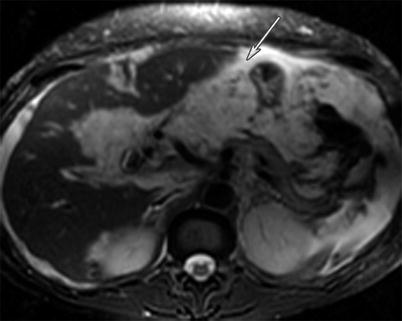
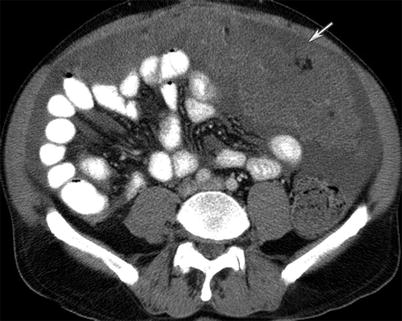
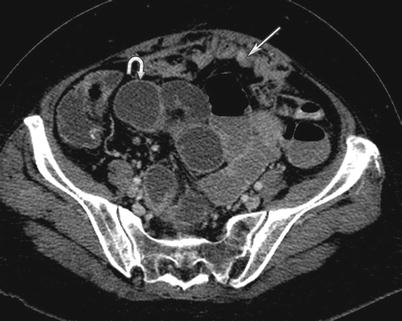

Fig. 16.4
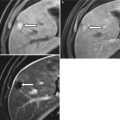
A 49-year-old female with gallbladder cancer. T2-weighted MRI image of the abdomen reveals peritoneal carcinomatosis in the form of widespread peritoneal thickening and masses encasing the liver surface with associated ascites (white arrow). The carcinomatosis is high in T2

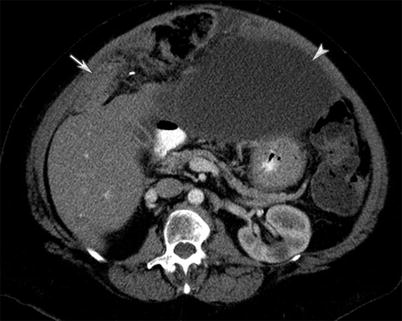
Fig. 16.5
A 58-year-old male with appendiceal/cecal mucinous carcinoma. CT of the abdomen and pelvis with intravenous and gastrointestinal contrast demonstrates omental thickening characteristic of omental caking (white arrow). Ascites is also present

Fig. 16.6
A 64-year-old female with high-grade serous carcinoma of the ovary. CT of the abdomen and pelvis with intravenous contrast reveals fluid-filled and dilated small bowel consistent with small bowel obstruction (curved arrow). The transition point of the bowel contains wall thickening from serosal metastatic deposits (not shown). Peritoneal carcinomatosis evidenced by omental thickening (straight arrow)
CT imaging is widely used for imaging of peritoneal metastasis. However, Low et al. has found that delayed gadolinium-enhanced MRI is accurate for detecting small volume peritoneal tumors and carcinomatosis [3]. Metastatic disease is suspected when peritoneal enhancement is greater than that of the liver on delayed images (5–10 min) with associated peritoneal nodules, thickening, and masses. Also, adding DWI improves the sensitivity and specificity for depicting peritoneal metastasis [3].
Peritoneal Thickening/Metastasis from Mucinous Disease
The term pseudomyxoma peritonei is not a pathologic diagnosis, but a clinical and radiographic description. It is described when there is abundant, thick, and gelatinous material on the surface of the peritoneum and associated viscera, without invasion of underlying tissues. This is usually due to low-grade mucinous carcinomas arising from the appendix (Fig. 16.7). On the other hand, mucinous carcinomatosis usually arises from a mucinous carcinoma from other parts of the gastrointestinal tract, gallbladder, pancreas, or ovary. In contrast to pseudomyxoma peritonei, mucinous carcinomatosis often has larger soft tissue and fibrotic components and is characterized by a more aggressive course, such as invasion into the adjacent organs, causing omental carcinomatosis, containing mesenteric and retroperitoneal lymphadenopathy, and producing pleural effusions and hematogenous metastasis elsewhere (Fig. 16.8). Occasionally, it will be difficult to distinguish pseudomyxoma peritonei from mucinous carcinomatosis, as their imaging features can overlap.
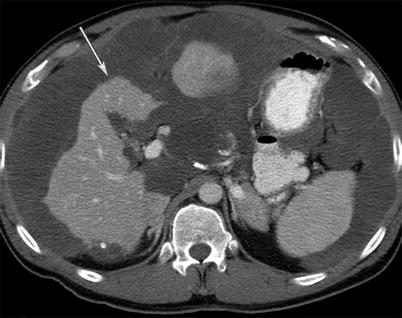
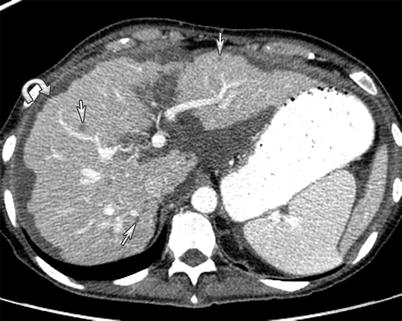

Fig. 16.7
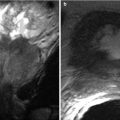
A 54-year-old male with primary appendiceal mucinous adenocarcinoma. Pseudomyxoma peritonei seen as thick and gelatinous material filling the peritoneum and scalloping the surface of the viscera such as the liver without invasion (arrow) on CT imaging of the abdomen with intravenous contrast

Fig. 16.8
A 49-year-old female with mucinous adenocarcinoma of the colon. Mucinous carcinomatosis evident in the form of mucinous encasement and scalloping of the liver capsule (curved arrow) and liver parenchymal metastasis in the form of sub-centimeter hypodense new nodules (arrows) as seen on CT of the abdomen with intravenous and gastrointestinal contrast
CT will show mucinous ascites as low in attenuation with scalloping of the liver, spleen, and gastric surfaces. Unlike regular ascites, mucin will displace bowel and mesentery and causing them to not float.
Peritoneal Thickening/Metastasis from Peritoneal Lymphomatosis
When there is diffuse peritoneal thickening, peritoneal lymphomatosis can be included in the differential diagnosis. Gastrointestinal lymphoma can present with omental caking, peritoneal thickening, and masses as seen on CT (Fig. 16.9). The classic appearance has been described as the “sandwich sign,” in which confluent masses encase the superior mesenteric artery and vein (Fig. 16.10). A thickened bowel wall with aneurismal dilatation and splenomegaly can help in differentiating peritoneal lymphomatosis from peritoneal carcinomatosis [4].
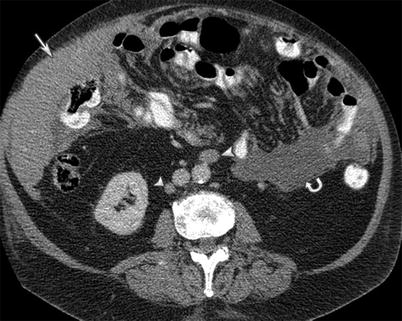
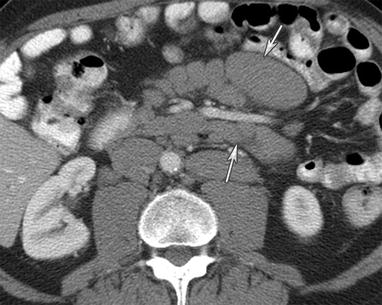

Fig. 16.9
A 77-year-old male with mantle cell lymphoma. CT of the abdomen with intravenous and gastrointestinal contrast reveals omental caking (arrow) and ascites (curved arrow) from peritoneal lymphomatosis. The clue to the diagnosis is the retroperitoneal lymphadenopathy (arrowhead)

Fig. 16.10
A 67-year-old male with chronic lymphocytic leukemia with Richter’s transformation. CT of the abdomen with intravenous and gastrointestinal contrast shows multiple mesenteric lymphadenopathy encasing the mesenteric vessels, known as the “sandwich sign” (arrows)
Peritoneal Thickening/Metastasis from Peritoneal Sarcomatosis
Gastrointestinal stromal tumor (GIST) is a mesenchymal tumor that typically arises from the gastrointestinal tract. They are rarely seen in the omentum and mesentery. Their presentation as seen on CT imaging can be diffuse peritoneal thickening and seeding, similar to peritoneal carcinomatosis. They have been found to characteristically present as large masses with hemorrhage or necrosis. Furthermore, a differentiating feature that has been described is their hypervascularity (Fig. 16.11) [4].
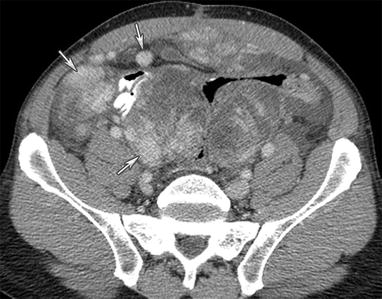

Fig. 16.11
A 59-year-old male with gastrointestinal stromal tumor. CT of the abdomen and pelvis with intravenous and gastrointestinal contrast demonstrates multiple enhancing nodules and masses throughout the peritoneum (arrows) suggestive of peritoneal sarcomatosis
Peritoneal Thickening from Primary Peritoneal Tumors
In the absence of a visceral or malignant tumor elsewhere, primary peritoneal tumors can present with peritoneal thickening and masses. Primary peritoneal tumors are rare. Examples include malignant mesothelioma, primary peritoneal serous carcinoma, multicystic mesothelioma, leiomyomatosis peritonealis, and desmoplastic small round cell tumor. They arise from mesothelial, submesothelial, and uncommitted stem cells [1].
Malignant mesotheliomas can arise in the pleura, pericardium, and peritoneum, where the cavities are lined by a serosal membrane. They have also been found in the tunica vaginalis, since it is a continuation of the peritoneal membrane. The majority of malignant mesotheliomas involve the pleura. 6–10 % involves the peritoneum [1]. Malignant mesothelioma is known to be associated with asbestos exposure, particularly in men where the majority is found.
Two patterns of peritoneal involvement have been described on cross-sectional imaging by malignant mesothelioma. They can be seen as focal intraperitoneal masses or diffuse involvement of the peritoneum. The focal pattern presents as solid, heterogenous masses with irregular margins. Invasion of the adjacent visceral organs can occur.
The diffuse pattern encases the peritoneal cavity and the viscera in a rind. The peritoneum is thickened and nodular. Involvement of the mesentery has been described as pleated or stellate. Omental caking and ascites can occur, sometimes overlapping and being indistinguishable from peritoneal carcinomatosis. Nevertheless, when there are imaging findings of prior asbestos exposure and sheetlike thickening of the peritoneum, malignant mesothelioma should be highly considered (Fig. 16.12).
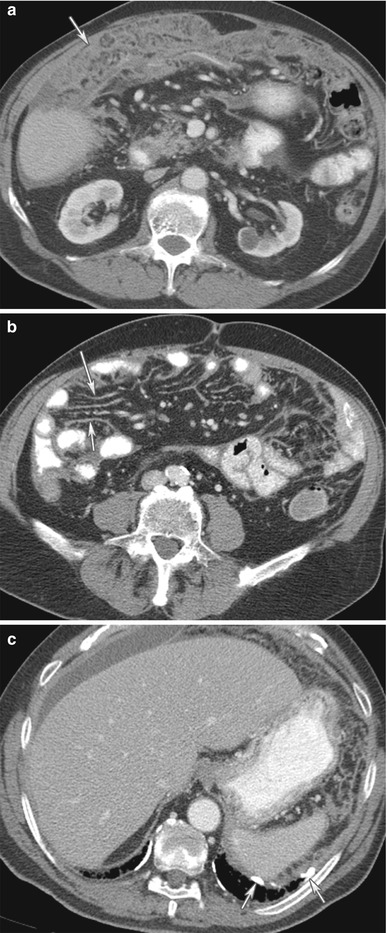

Fig. 16.12
(a–c) An 80-year-old male with malignant mesothelioma. CT of the abdomen with contrast reveals omental thickening from malignant mesothelioma (arrow) (a). There is sheetlike thickening of the mesentery from malignant mesothelioma (arrows) (b). Additional, calcified pleural thickening (arrows) from prior asbestos exposure, a clue to the diagnosis of malignant mesothelioma (c)
Primary peritoneal serous carcinoma is also another entity that should be considered in the differential diagnosis of primary peritoneal tumors presenting as peritoneal thickening, especially in a female patient with no visceral primary or ovarian mass. Primary peritoneal serous carcinoma spawns from the peritoneal epithelium, resembling malignant ovarian surface epithelial tumor. It is also indistinguishable from metastatic serous ovarian carcinoma at gross, histologic, and immunohistochemical examination.
Nonspecific cross-sectional imaging characteristics include ascites and peritoneal and omental nodules and masses (Fig. 16.13). Nevertheless, a few criteria have been established for the diagnosis of primary peritoneal serous carcinoma:
1.
Both ovaries are normal.
2.
The involvement of extraovarian sites must be greater than the surface of both ovaries.

Fig. 16.13
A 70-year-old female with primary peritoneal serous carcinoma. CT of the abdomen and pelvis with intravenous and gastrointestinal contrast reveals omental thickening (arrow) and ascites (arrowhead). The imaging findings are not specific for primary peritoneal serous carcinoma
Peritoneal Thickening from Infection/Inflammation
Besides cancer, an inflammatory or infectious process can also cause peritoneal thickening (Fig. 16.14). Granulomatous peritonitis has been used to describe a spectrum of infection and inflammation in the peritoneum, including tuberculosis, histoplasmosis, and pneumocystic infection; foreign materials such as talc and barium; bowel contents; ruptured ovarian cysts; bile; gallstones; Crohn disease, sarcoidosis, and Whipple disease. Splenic or lymph node calcification along with thickening of the cecum and terminal ileum and low attenuation lymphadenopathy may help in identifying tuberculosis causing peritoneal thickening (Fig. 16.15).
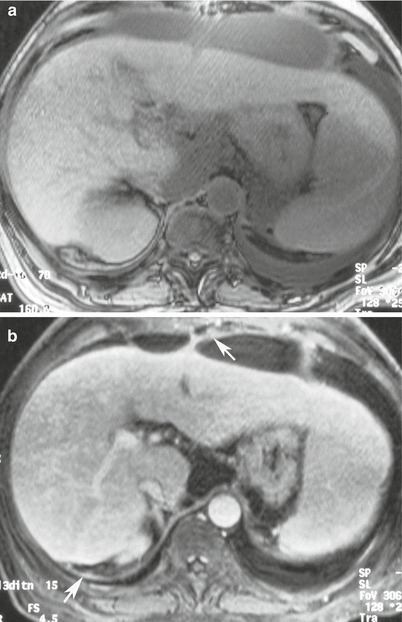
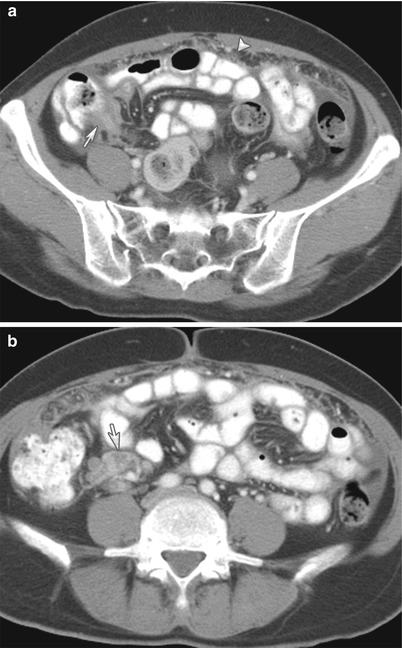

Fig. 16.14
MRI of the abdomen with T1 pre-intravenous (a) and post-intravenous gadolinium contrast (b) demonstrates thickening and enhancement from acute peritonitis (arrows)

Fig. 16.15
(a, b) A 60-year-old female with granulomatous peritonitis from tuberculosis. CT of the abdomen and pelvis with intravenous and gastrointestinal contrast reveals wall thickening and inflammation near the cecum (arrow) and omental nodularity (arrowhead) (a). Additionally, a cluster of low-density enlarged lymph nodes congregate in the ileocecal region (arrow), (b)
Peritoneal thickening found in a peritoneal dialysis patient could suggest sclerosing encapsulating peritonitis, a rare entity. It is also referred as abdominal cocoon. It is a rare complication observed in long-term dialysis patients and other forms of peritoneal inflammation such as peritoneovenous or ventriculoperitoneal shunts, tuberculosis, and fibrogenic foreign material. The disease manifests in the form of peritoneal thickening with signs of inflammation. Additionally, peritoneal thickening, peritoneal calcifications, loculated fluids, small bowel feces sign, small bowel obstruction, and signs of bowel ischemia have been described [5]. Classically, an abdominal cocoon manifests as a collection of obstructed bowel loops surrounded by a thick fibrocollagenous membrane/sac as seen on CT imaging (Fig. 16.16).
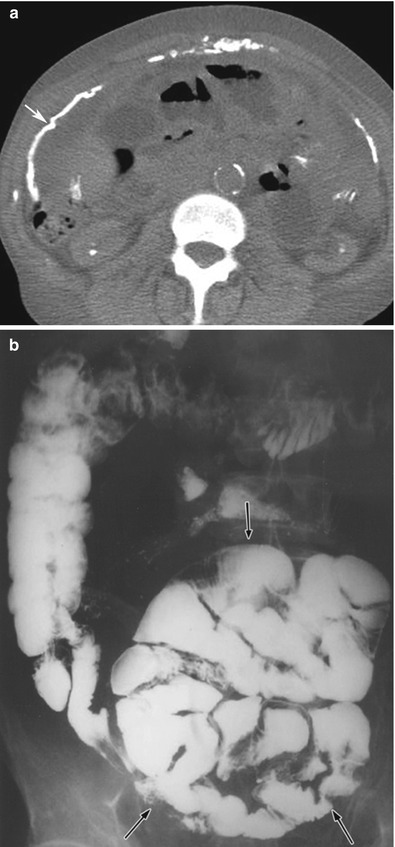

Fig. 16.16
(a, b) CT of the abdomen (a) without intravenous contrast in a patient with sclerosing encapsulating peritonitis shows peritoneal thickening and calcification (arrow). Note that some of the bowel loops are also dilated. The dilated, tethered, and obstructed bowel loops are encased within a fibrotic peritoneum, as shown on a small bowel series (black arrows on b). This is the typical appearance of an abdominal cocoon
Peritoneal Thickening/Masses from Miscellaneous Tumors and Tumorlike Lesions
Miscellaneous etiologies such as endometriosis, splenosis, gliomatosis peritonei, and osseous metaplasia can also cause peritoneal masses and thickening.
Splenosis is the ectopic implantation of splenic tissue after disruption of the normal splenic capsule. It can be located anywhere in the peritoneal cavity. Splenosis should be considered when a peritoneal mass or nodule demonstrates similar echogenicity to the spleen on ultrasound and similar attenuation, signal intensity, and enhancement on CT or MRI, respectively (Fig. 16.17). Equivocal lesions can be further investigated with sulfur colloid or technetium 99 m heat-damaged tagged red blood cell scintigraphy. The ectopic splenule will take up the radiotracer.
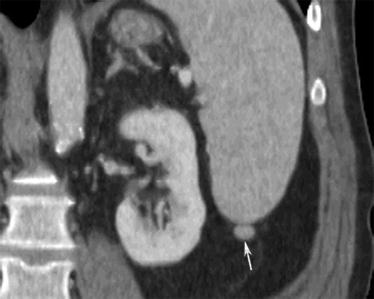

Fig. 16.17
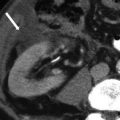
A 53-year-old male with colon cancer. CT of the abdomen with intravenous contrast in the coronal view shows a small nodule in the left paracolic gutter inferior to the spleen (arrow). It has the same enhancement characteristic and attenuation as the spleen, suggesting a splenule and not metastatic deposit
Another example of peritoneal thickening and mass formation in the peritoneum is from endometriosis. Endometriosis occurs when there is functional endometrial glands and stroma outside the uterus. Not only the pelvis but the remainder of the abdominal peritoneum can be involved. These ectopic endometrial implants occur commonly in women.
The ultrasound and CT findings of endometriosis are nonspecific. They can be solid, cystic, or complex cystic and solid changes as seen on CT imaging. MRI is more specific for the diagnosis of endometriosis, appearing hyperintense on T1-weighted and hypointense on T2- weighted images due to blood products (Fig. 16.18). Other features of MRI findings that have been described include low signal intensity rim on T1- and T2-weighted images due to surrounding fibrosis and hemosiderin macrophages [2].
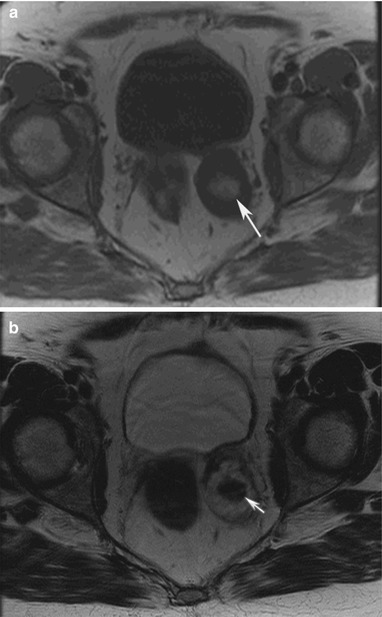

Fig. 16.18
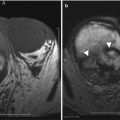
A 68-year-old female with history of metastatic islet cell tumor. MRI T1-weighted image of the pelvis reveals a left adnexal mass with central T1 hyperintensity (arrow) (a). The T2-weighted image reveals the mass contains central T2 hypointensity (arrow) (b). The findings are suggestive of blood products from endometrioma, confirmed with biopsy
Omental Masses
Occasionally, more focal pathologic conditions involve solely the omentum. Conditions such as acute epiploic appendagitis and omental infarction can be found not uncommonly. On the other hand, only a handful of cases have been described in the English literature for primary liposarcoma of the omentum. It is truly a rare condition. Other conditions such as metastatic disease and mesothelioma must also be considered (Algorithm 16.2).
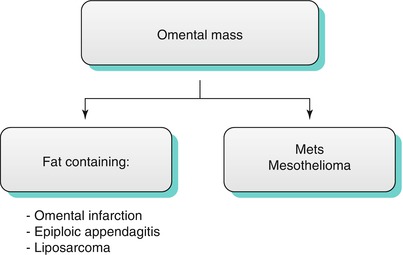

Algorithm 16.2 Differential diagnosis of common omental masses
Epiploic appendages are peritoneal pouches that arise from the colonic serosa, attached to the colon by a vascular stalk and comprised of fat. They are not seen normally by cross-sectional imaging, but can be identified when inflamed. Acute appendagitis has been understood to occur when it is torsed, resulting in vascular or venous occlusion leading to ischemia. Other causes include hernia incarceration, intestinal obstruction, and intraperitoneal loose body [6]. Furthermore, it has been associated with obesity, hernia, and unaccustomed exercise. It is a self-limited condition in most cases.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree