Images of the shoulder after surgery can be difficult to interpret. There are a multitude of procedures that are performed and the patient, or even the referring doctor, may not be familiar with the specific procedure that was undertaken. A patient returning with recurrent pain may have a complication of the procedure, from breakdown of repair, degeneration, or a new injury. It is important for the radiologist to assist the referring clinician in delineating the cause. To do this, one must be familiar with advantages and disadvantages of available modalities as well as the basic types of procedures, their imaging characteristics, and potential complications. This Chapter discusses procedures that are commonly encountered.
Use of Imaging Modalities
Radiographs
Radiographs should precede any advanced imaging after shoulder surgery. Baseline anteroposterior (internal and external rotation) and outlet (or “Y”) views are routinely used as a baseline after surgery. An axillary view and Grashey view (scapular anteroposterior, oriented tangential to the glenohumeral joint) are commonly added. Loosening of components can be seen as frank displacement or more subtle lucency around the implant indicative of motion. If advanced imaging is required, surgical history and radiographs can be used to estimate whether significant artifact is anticipated, thus helping determine whether ultrasound, computed tomography (CT), or MRI is best (Table 8-1). Conventional arthrography may be useful for evaluation of prostheses or intra-articular implants suspicious for loosening; contrast extending around the implant is indicative of pathology. It should be noted that communication through the repaired rotator cuff is not necessarily pathologic, so this finding on a conventional arthrogram may be clinically inconsequential. However, arthrography combined with cross-sectional imaging (CT or MRI) offers more comprehensive analysis of the anatomy corresponding to contrast communication and can be extremely useful in the postoperative setting.
Table 8-1 Anticipated metal artifact on MRI and recommendations | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Magnetic Resonance Imaging
MRI has been used increasingly for evaluation of the postoperative shoulder because it can provide a global assessment, evaluating the numerous potential causes of recurrent or new symptoms after surgery. However, interpretation is made more challenging by variable degrees of susceptibility artifact. Artifact on MRI is related to a number of factors, including metal type, size, and surface complexity. In general, the more complex the metallic surface, the more artifact is seen. Type of metal also has an effect with titanium causing relatively little artifact and stainless steel and cobalt chrome creating a more severe effect. To a certain extent, the effect on MRI can be predicted, although burring (like with acromioplasty) leaves behind microscopic metal fragments
with very complex surfaces, invisible on radiographs, which can leave the MR examination almost uninterpretable. Metal anchors cause artifact but are small enough such that the protocol usually does not need to be altered. Radiolucent bioabsorbable implants, commonly used in current practice, cause little to no artifact on MRI. If artifact is problematic, there are protocol changes that can be useful (1). This includes avoiding use of gradient-echo sequences that enhance artifact resulting from T2* susceptibility effects. Use of spin-echo sequences with a relatively lower TE, higher bandwidth, larger field of view, and higher matrix can significantly reduce metal artifact compared with a standard protocol. In general, fat suppression using selective fat presaturation is prone to accentuation of artifact if there is significant metal present resulting from field inhomogeneity, and simply removing fat suppression can improve image quality. Short tau inversion recovery (STIR) is a good alternative for obtaining fluid-sensitive information with fat suppression. Also, if artifact from an implant is asymmetrically shaped, simply swapping phase and frequency can often recover adjacent tissue signal (Table 8-2).
with very complex surfaces, invisible on radiographs, which can leave the MR examination almost uninterpretable. Metal anchors cause artifact but are small enough such that the protocol usually does not need to be altered. Radiolucent bioabsorbable implants, commonly used in current practice, cause little to no artifact on MRI. If artifact is problematic, there are protocol changes that can be useful (1). This includes avoiding use of gradient-echo sequences that enhance artifact resulting from T2* susceptibility effects. Use of spin-echo sequences with a relatively lower TE, higher bandwidth, larger field of view, and higher matrix can significantly reduce metal artifact compared with a standard protocol. In general, fat suppression using selective fat presaturation is prone to accentuation of artifact if there is significant metal present resulting from field inhomogeneity, and simply removing fat suppression can improve image quality. Short tau inversion recovery (STIR) is a good alternative for obtaining fluid-sensitive information with fat suppression. Also, if artifact from an implant is asymmetrically shaped, simply swapping phase and frequency can often recover adjacent tissue signal (Table 8-2).
Table 8-2 Changes in MRI protocol and usefulness for reducing metal artifact | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||
Direct Magnetic Resonance Arthrography
Although noncontrast MRI has been shown to be accurate in diagnosing recurrent pathology in the postoperative shoulder (2), experience at our institution with direct MR arthrography (MRA) has shown that it can provide improved diagnostic capability over noncontrast MRI and it improves reader confidence and reduces the radiologist’s learning curve (3–5). One of the advantages of direct MRA is that by injecting contrast into the joint, there is distention of the joint capsule and separation of the capsular structures from the labrum allowing for better visualization. Additionally, fluid is forced through or around pathologic lesions in the labrum or rotator cuff allowing for improved sensitivity in detecting these lesions. Another advantage of direct MRA is that it has a higher signal-to-noise ratio compared with noncontrast MRI resulting from the use of T1 weighting, which has less noise compared with T2 or STIR techniques. Higher SNR with direct MRA provides improved contrast resolution, which helps define small structures. The appropriate volume of contrast that should be injected depends on the type of surgery that was performed. For labral/capsular tears, the volume of injection should be kept low (usually 12 mL is adequate) to avoid overdistention, which increases the chance of extracapsular leakage. For rotator cuff repair/impingement surgery, the volume of injection should be maximized for the
purpose of distending the joint capsule to its end point and forcing contrast through defects in the cuff. Injection is performed until capsular resistance is reached or until pain prevents full distention, which typically occurs after injection of 16 mL if there is no cuff defect. Note should be made that postoperative scarring of the subacromial/subdeltoid bursa can prevent contrast extension. A 2- to 3-mmol solution of gadolinium contrast is optimal; this can be prepared in a 1:200 dilution with saline (i.e., 1 mL of gadolinium in 200 mL of saline). If there is a delay expected before imaging is to be performed, the syringe can be rinsed with 1:1000 epinephrine before the procedure before the gadolinium is drawn up to constrict synovial vessels and reduce the rate of joint fluid resorption.
purpose of distending the joint capsule to its end point and forcing contrast through defects in the cuff. Injection is performed until capsular resistance is reached or until pain prevents full distention, which typically occurs after injection of 16 mL if there is no cuff defect. Note should be made that postoperative scarring of the subacromial/subdeltoid bursa can prevent contrast extension. A 2- to 3-mmol solution of gadolinium contrast is optimal; this can be prepared in a 1:200 dilution with saline (i.e., 1 mL of gadolinium in 200 mL of saline). If there is a delay expected before imaging is to be performed, the syringe can be rinsed with 1:1000 epinephrine before the procedure before the gadolinium is drawn up to constrict synovial vessels and reduce the rate of joint fluid resorption.
Indirect Magnetic Resonance Arthrography
Indirect MRA is performed by intravenous administration of the standard dose of gadolinium (0.1 mmol/kg) followed by delayed MRI (after approximately 30 minutes). The intravenous contrast is taken up into the joint fluid, providing an arthrographic effect. However, there is no distention of the joint capsule (unless the patient already had a joint effusion), which may reduce the sensitivity for detecting cuff or labral pathology. Signal–to-noise ratio is also lower with indirect MRA and postoperative granulation tissue may enhance, which makes evaluation of the surgical site difficult. Despite its limitations, indirect MRA can be a useful alternative if the patient refuses intra-articular injection or if logistic considerations prevent performance of direct MRA.
Multidetector Computed Tomography
CT is widely available and relatively low-cost compared with MRI. It is also very useful for evaluation of the postoperative shoulder after certain procedures. Noncontrast multidetector CT (MDCT) yields high-quality images in any plane through isotropic data sets and reformatting capability, even in the presence of large metal implants (6). Consequently, for evaluation of complications (loosening, displacement, fracture) associated with intramedullary rods, cortical plates, screws, and large anchors, MDCT is optimal. Metal streak artifact is minimized by using a higher voltage (140 kVp), higher exposure (200–400 mAs), and reduced pitch with slice overlap (less than 1). These measures result in higher photon flux through the patient and improve image quality; however, these steps also result in increased radiation dose.
Computed Tomography Arthrography
MDCT can be combined with administration of intra-articular iodinated contrast (full-strength is typically used) to achieve a CT arthrogram. This is an excellent technique for detection of labral or rotator cuff retear, especially when significant metal is present. However, it should be recognized that as a result of low tissue contrast, diagnoses can only be made when intra-articular contrast bathes the area of concern. For example, a partial-thickness rotator cuff retear on the bursal side will not be detected on a CT arthrogram. Also, contrast fills in defects but will not detect pathologic scar tissue that contrast does not penetrate.
Ultrasound
Ultrasound can be very useful in some situations (7). It is widely available but demands requisite expertise and proper equipment (e.g., high-frequency probes). Also,
patients with limited range of motion may not tolerate positioning required to optimally evaluate the tendons. Nevertheless, because anchors are set within bone and create no artifact, ultrasound is an attractive option for diagnosing rotator cuff retear. In fact, anchors may be visible only as small depressions at the greater tuberosity and can even be overlooked, appearing similar to herniation pits and cortical irregularity commonly present (Fig. 8.1). A corollary of this is that displaced implants (indicating breakdown of repair) are easily detected. On the other hand, ultrasound is disadvantageous for evaluating labral repair and most intra-articular (as well as intraosseous) pathology.
patients with limited range of motion may not tolerate positioning required to optimally evaluate the tendons. Nevertheless, because anchors are set within bone and create no artifact, ultrasound is an attractive option for diagnosing rotator cuff retear. In fact, anchors may be visible only as small depressions at the greater tuberosity and can even be overlooked, appearing similar to herniation pits and cortical irregularity commonly present (Fig. 8.1). A corollary of this is that displaced implants (indicating breakdown of repair) are easily detected. On the other hand, ultrasound is disadvantageous for evaluating labral repair and most intra-articular (as well as intraosseous) pathology.
Impingement/Rotator Cuff Surgery
Acromioplasty and Clavicular Resection
Acromioplasty is a procedure that involves smoothing the undersurface of the acromion, usually with a burr (Fig. 8.2). It is commonly performed for the treatment of primary impingement and can be performed with an open approach (typically anterolateral through a deltoid split) or arthroscopically through a posterior approach (8). This is often combined with other procedures, including the Mumford procedure, which involves resection of the distal clavicle and/or rotator cuff repair.
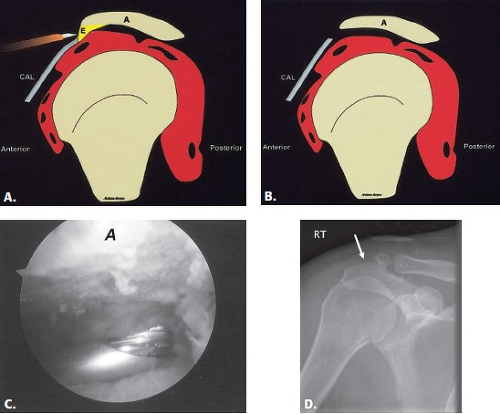 Figure 8.2 A–B: Diagrammatic representation of an acromioplasty procedure. The subacromial spur is resected and the undersurface of the acromion is shaved with a burr so that no bony prominence remains. An anterior approach is used for open procedures and a posterior approach is used for the arthroscopic approach. A, acromion; CAL, coracoacromial ligament; E, enthesophyte. (From Mohana-Borges AVR, Chung CB, Resnick D. MR imaging and MR arthrography of the postoperative shoulder: spectrum of normal and abnormal findings. Radiographics. 2004;24:69–85 [37] with permission.) C: Arthroscopic image shows burr instrument shaving acromial undersurface. (Image courtesy of Joseph Abboud, MD, Philadelphia, Pa.) D: Frontal radiograph of the shoulder with deficiency of the anterolateral acromion (arrow) representing prior acromioplasty. |
The bone burring used in acromioplasty usually results in significant MR artifact. This can make visualization of acromial morphology difficult; however, it does not usually prevent adequate rotator cuff evaluation. The presence of fluid in the subacromial/subdeltoid bursa is common and does not necessarily indicate symptomatic bursitis. This fluid can be present for years after surgery and may enhance after administration of intravenous contrast. However, if the amount of fluid is prominent, correlation for symptoms of recurrent impingement, subacromial pain, or crepitus on range of motion should be made.
In patients with postoperative symptoms, recurrent subacromial impingement may occur in a delayed fashion as a result of reformation of spurs. It can also occur as a result of incomplete or overaggressive acromioplasty. Therefore, the acromial morphology should be evaluated in the postoperative setting. Surgeons burr off the subacromial spur and attempt to make the undersurface flat; if there is a residual lateral point or angle at the midportion (Figs. 8.3 and 8.4), recurrent impingement may occur. On the other hand, excessive acromioplasty can result in instability. If the artifact from the burring is too extensive, CT can be performed to better evaluate the acromial morphology. An unrecognized os acromiale can also lead to recurrent impingement. Pseudoarthrosis
of an os acromiale may also be treated by fixating the ossicle with the acromion using a screw (Fig. 8.5).
of an os acromiale may also be treated by fixating the ossicle with the acromion using a screw (Fig. 8.5).
 Figure 8.5 Fixation of os acromiale. Frontal radiograph shows two fixation screws used to treat a symptomatic os acromiale with pseudarthrosis. |
The coracoacromial ligament is often lysed during acromioplasty; however, currently, surgeons try to preserve this ligament or reattach it after acromioplasty. Comment should be made regarding whether this structure is intact or lysed and whether it is thickened. Sagittal MR images facilitate this evaluation.
The Mumford procedure (resection of the distal clavicle) is usually performed in patients with symptomatic acromioclavicular osteoarthritis, and it may be performed in conjunction with acromioplasty (Fig. 8.6). This usually causes little artifact, and the appearance may be mistaken for an old acromioclavicular joint separation. The best imaging plane for this evaluation is the axial plane.
 Figure 8.6 Mumford procedure. A–B: Diagrammatic representation (A, acromion; C, clavicle) showing resection of the distal clavicle. Resultant widening of the acromioclavicular interval can be mistaken for traumatic acromioclavicular joint separation. (From Mohana-Borges AVR, Chung CB, Resnick D. MR imaging and MR arthrography of the postoperative shoulder: spectrum of normal and abnormal findings. Radiographics. 2004;24:69–85 [37] with permission.) C: Axial gradient-recalled echo (GRE) MR image. Note the presence of susceptibility artifact (arrows) from the procedure, which is generally minimal. |
Rotator Cuff Repair
Surgical approaches for rotator cuff repair include open, miniopen, and arthoscopic (9–11). Open surgery involves taking the anterior deltoid down from the
acromion. This approach offers the best exposure but prolongs recovery time and can lead to breakdown (“dehiscence”) at the site of deltoid reattachment. The miniopen technique involves an arthroscopic acromioplasty and rotator cuff repair through a vertical split in the deltoid muscle. This speeds recovery but offers limited exposure. Arthroscopic repair potentially allows the fastest rehabilitation, but it can be technically challenging to repair large tears or to place a double row of sutures.
acromion. This approach offers the best exposure but prolongs recovery time and can lead to breakdown (“dehiscence”) at the site of deltoid reattachment. The miniopen technique involves an arthroscopic acromioplasty and rotator cuff repair through a vertical split in the deltoid muscle. This speeds recovery but offers limited exposure. Arthroscopic repair potentially allows the fastest rehabilitation, but it can be technically challenging to repair large tears or to place a double row of sutures.
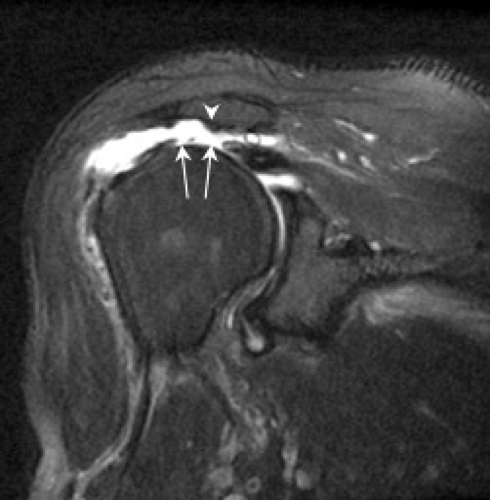
There are different types of rotator cuff repair surgeries, including simple débridement of granulation tissue at a partial-thickness articular surface or bursal-sided tear, tendon-to-tendon suturing (in the case of a longitudinal tear), or tendon-to-bone repair of a full-thickness tear (Fig. 8.7). In tendon-to-bone repair, suture anchors are placed in the tuberosity and the end of the cuff tendon is tied down (Fig. 8.8). The surgeon may “rough up” the bony surface to facilitate healing of the tendon attachment. Historically, the procedure involved placing a single row of suture anchors along the tear site (Fig. 8.9); more recently, it has been recognized that the cuff tendons insert on a “footprint” or area, not a single point. Therefore, more surgeons are reattaching the tendon with a double row of sutures (Fig. 8.10). Knowledge of the type of rotator cuff surgery may facilitate interpretation of MR images; the location of suture artifact on MR images may also be helpful, indicating the type of repair. For example, the presence of artifact at the tendon substance indicates a tendon-to-tendon repair, whereas if the artifact is at the greater tuberosity, it indicates tendon-to-bone repair. Artifact is usually minimal and does not limit the diagnostic information of the MR images. A repaired tendon should normally be low in signal on T2-weighted images (Fig. 8.11), similar to normal tendon, although there may be residual mild intermediate signal from tendinosis or curvilinear high signal related to suture artifact (12–14). The repaired tendon may appear thickened or thinned and may demonstrate an irregular-appearing margin, which does not necessarily imply a pathologic lesion. Scar tissue in the subacromial/subdeltoid bursa may be present, which can blend with cuff signal, making evaluation difficult.
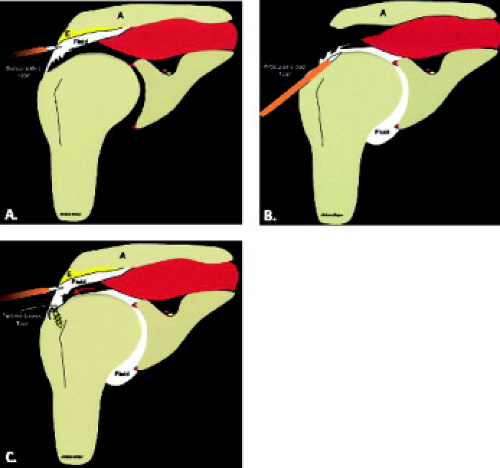 Figure 8.7 Diagrammatic representation of rotator cuff surgery. A, acromion; E, enthesophyte. A: Débridement of a partial-thickness bursal-sided tear. B: Débridement of a partial-thickness articular-sided tear. C: Repair of a full-thickness tear with a suture anchor placed at the greater tuberosity. (From Mohana-Borges AVR, Chung CB, Resnick D. MR imaging and MR arthrography of the postoperative shoulder: spectrum of normal and abnormal findings. Radiographics
Get Clinical Tree app for offline access
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|