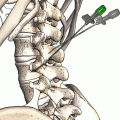
Fig. 31.1
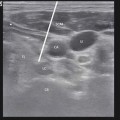
Engorged blood vessels in the epidural cavity as observed during epiduroscopy. Inserted in upper right corner is fluoroscopy showing location for epiduroscopy tip (left anterior border of L5)
The Diagnosis of Epidural Fibrosis (Adhesions)
As with any patient, a thorough musculoskeletal and neurologic examination should be performed. In addition to standard dural tension provocative tests, we recommend a provocative test called “dural tug.” To perform the test, the patient should be made to sit up with a straight leg, bend forward until their back pain starts to become evident, and rapidly flex the head and neck forward. During this maneuver, the dura is stretched cephalad and if adhered to structures such as the posterior longitudinal ligament, the most heavily innervated spinal canal structure, the movement of the dura will elicit back pain that is localized to the pain generator. The dural tug maneuver is no longer provocative after percutaneous neuroplasty (Fig. 31.2a–e).
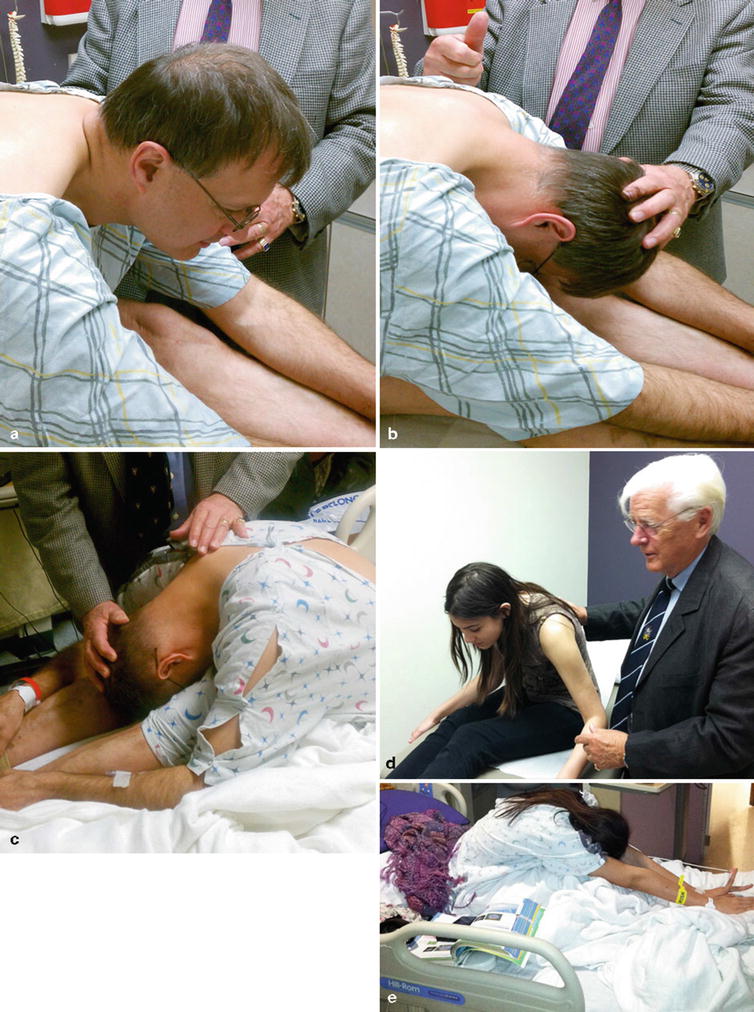

Fig. 31.2
(a) The “dural tug” maneuver being performed prior to percutaneous neuroplasty. (b) Note pain reproduction prior to full neck flexion secondary to dural adhesions. (c) Patient after percutaneous neuroplasty with pain-free neck and back flexion due to treatment of dural adhesions. (d) There is decreased spine flexion prior to treatment secondary to dural adhesions. (e) After treatment, the same patient demonstrates increased painless flexion of the spine
Imaging modalities with utility in the diagnosis of epidural fibrosis include MRI, computed tomography (CT), and epiduroscopy; however, the best way to diagnosis epidural fibrosis is through an epidurogram [13–16]. In contrast to MRI or CT, an epidurogram is able to demonstrate filling defects which can be correlated with a patient’s symptoms in real time. The sensitivity and specificity of MRI and CT for detecting epidural fibrosis are 50 and 70 %, respectively [17].
Patient Selection
There are many conditions for which the lysis of adhesions procedure may be appropriate for. The most common indication is for neck or back pain and radiculopathy secondary to postsurgical epidural fibrosis. Other indications include disk disruption, metastatic carcinoma of the spine leading to compression fracture, multilevel degenerative spondylosis, spinal stenosis, and pain unresponsive to spinal cord stimulation and spinal opioids [18].
Contraindications
Absolute contraindications to adhesiolysis include sepsis, chronic infection, coagulopathy, local infection at procedure site, syrinx formation, and patient refusal. Although not an absolute contraindication, arachnoiditis poses significant risk when performed by interventional pain physicians with limited experience with adhesiolysis. In arachnoiditis, the risk of loculation and subdural or subarachnoid spread is significantly increased; it is for this reason that we suggest the referral of these patients to a more experienced physician if doubt should arise.
Benefits and Risks
In order to obtain informed consent, risks and benefits should be discussed with the patient prior to performing adhesiolysis. Potential benefits of the procedure include pain relief, increased function, and the possible reversal of neurologic symptoms. Risks include, but are not limited to, bruising, bleeding, infection, reaction to medications used (i.e., hyaluronidase, contrast, local anesthetic, corticosteroids, hypertonic saline), damage to nerves or blood vessels, no or little pain relief, bowel/bladder incontinence, worsening of pain, and paralysis. In our practice, we have never seen an allergic reaction to hyaluronidase and neither was any reported on a survey of large groups of ophthalmic anesthesiologists in its use with retrobulbar blocks [28]. Additionally, patients with a history of urinary incontinence should have urodynamic evaluation by a urolo-gist before the procedure to document the preexisting urodynamic etiology and pathology.
Anticoagulant Medication
Any medication that prolongs bleeding and/or clotting should be held prior to performing adhesiolysis. We suggest contacting the physician prescribing the anticoagulant prior to holding it for adhesiolysis—typically the patient’s primary care physician or cardiologist. We suggest the following times to withhold anticoagulant or antiplatelet medications prior to adhesiolysis: nonsteroidal anti-inflammatory 4 days, aspirin 7–10 days, clopidogrel (Plavix) 7 days, ticlopidine (Ticlid) 10–14 days [19], warfarin (Coumadin) 5 days [18], subcutaneous heparin a minimum of 12 h, and low-molecular-weight heparin 24 h [19]. Nonprescription homeopathic medications that prolong bleeding should also be withheld for approximately 2 weeks. These include fish oil, vitamin E, Gingko biloba, garlic, ginseng, and St. John’s wort. We also routinely perform laboratory analysis as close to the day of the procedure as possible. Laboratory studies to ensure adequate coagulation status that we use include a complete blood count, prothrombin time, partial thromboplastin time, and a platelet function assay, or bleeding time.
Preoperative Laboratory Evaluation
In addition to the aforementioned coagulation parameters discussed, each patient should undergo a complete blood count and clean-catch urinalysis to rule out any underlying infection. As with all elective procedures, any abnormal laboratory value should warrant cancellation of adhesiolysis and further workup by the patient’s primary care physician.
Technique
Adhesiolysis is most routinely performed in the lumbar and caudal regions of the spine, but can also be performed in the cervical and thoracic regions. This chapter will provide detailed explanation of the caudal and lumbar transforaminal placement of catheters.
Adhesiolysis is performed under strict sterile conditions in the operating room with prophylactic broad-spectrum antibiotics given prior to the procedure and on postoperative day 1. We currently prefer cefazolin 1 g intravenously or clindamycin 600 mg intravenously for those allergic to penicillin. All procedures are performed with an anesthesiologist or nurse anesthetist providing monitored anesthesia care and/or appropriate sedation.
Lysis of Adhesions via a Caudal Catheter
The patient is placed in the prone position on the operating room table. Lumbar lordosis is minimized through the use of pillows placed under the abdomen. The patient’s low back and buttocks are sterilely prepped and draped. To begin the procedure, the sacral hiatus is identified through palpation or with AP fluoroscopic guidance. If using palpation, the sacral hiatus can be identified slightly caudal to the sacral cornu and mimics the feel of the area between two knuckles on the dorsum of the hand. A skin wheal should be made approximately 5 cm caudal and 2.5 cm lateral to the sacral hiatus over the contralateral buttock to the targeted nerve root. The distal approach is advocated to reduce the frequency of meningitis by effectively tunneling the catheter. The skin wheal is then superficially punctured with an 18-gauge needle; through the same puncture site, a 15- or 16-gauge RX Coudé 2 needle is inserted and directed toward the sacral hiatus. The RX Coudé 2 is initially advanced at a 45° angle via fluoroscopic guidance or by palpation of sacral hiatus with the left index finger (Figs. 31.3 and 31.4). Once through the sacral hiatus, the needle angle is then dropped to 30° and advanced under lateral fluoroscopic guidance into the caudal epidural space. AP fluoroscopic views should also be obtained to assure midline needle placement which will assist in directing the catheter. The needle should be advanced no further cephalad than the S3 level to prevent inadvertent dural puncture in patients with low-lying dura.
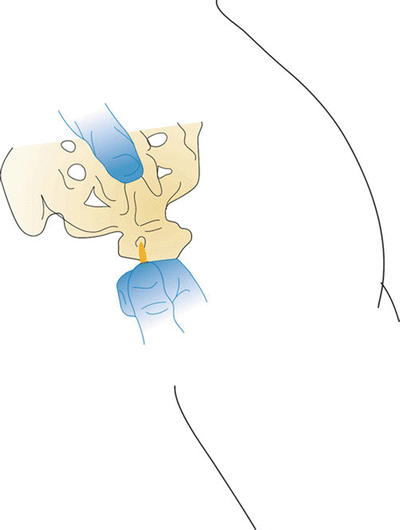
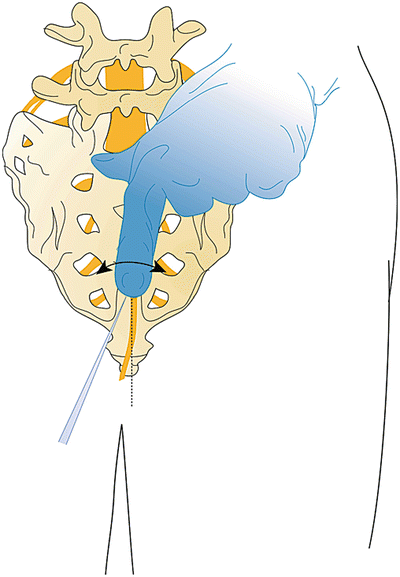

Fig. 31.3
Caudal lysis sequence—first find sacral hiatus and tip of coccyx

Fig. 31.4
Roll palpating index finger to identify the sacral cornua and thus the target sacral hiatus
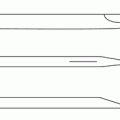
The RX Coudé 2 needle is used as opposed to other needles for several reasons including an angled tip, slightly blunted distal tip, and a non-cutting back edge of the needle opening. The non-cutting back edge of the needle opening allows for catheter manipulation with minimal risk of shearing. Tuohy needles have a cutting back edge of the needle opening which increases the risk of catheter shear.
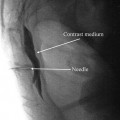
Once midline in the caudal epidural space, an epidurogram is performed using 10 mL of nonionic, water-soluble contrast. Omnipaque and Isovue are typically the contrast agents of choice and are suitable for myelography. Ionic water-insoluble contrast agents (Hypaque or Renografin) or ionic water-soluble contrast agents (Conray) must never be used. Injection of ionic contrast can result in seizures and death—inspect your contrast agent carefully prior to use. Additionally, aspiration prior to contrast injection should be performed to rule out intravascular or intrathecal injection.
Slowly inject the contrast agent and observe for filling defects. A normal epidurogram will have a “Christmas tree” pattern with the central canal being the trunk and the outline of the nerve roots making up the branches. An abnormal epidurogram will have areas where the contrast does not fill (Fig. 31.5). These are the areas of presumed epidural fibrosis and typically correspond to the patient’s radicular complaints. If vascular uptake is observed, the needle needs to be redirected.
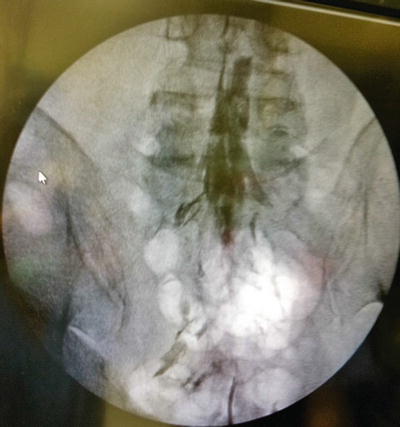

Fig. 31.5
Initial dye injection Omnipaque 240 (10 mL) in a patient with multilevel spinal stenosis showing filling defects bilaterally; pain worse on right side
After turning the distal opening of the needle ventral-lateral, insert a TunL Kath or TunL-XL-24 (stiffer) catheter with a bend on the distal tip through the needle (Fig. 31.6). The bend should be 1 in. from the tip of the catheter and at a 30° angle (Fig. 31.7). The bend will enable the catheter to be steered to the target level. Under continuous AP fluoroscopic guidance, advance the tip of the catheter toward the ventral-lateral epidural space of the desired level (Fig. 31.8). The catheter can be steered by gently twisting the catheter in a clockwise or counterclockwise direction. Avoid “propelling” the tip (i.e., twisting the tip in circles) because this makes it more difficult to direct the catheter. Do not advance the catheter up the middle of the sacrum because this makes guiding the catheter to the ventral-lateral epidural space more difficult. Ideal location of the tip of the catheter in the AP projection is in the foramen just below the midportion of the pedicle shadow (Figs. 31.9 and 31.10). Check a lateral projection to confirm that the catheter tip is in the ventral epidural space.
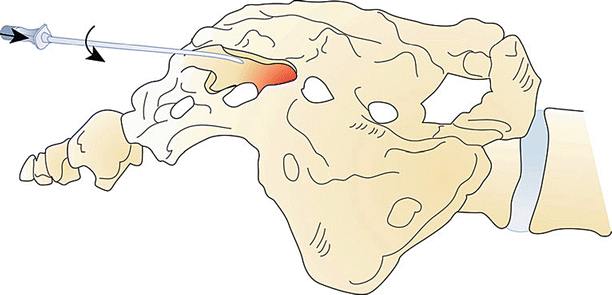
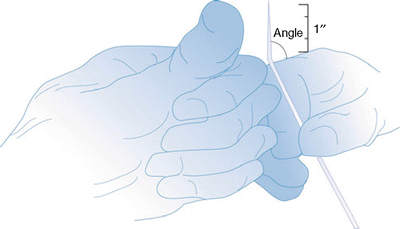
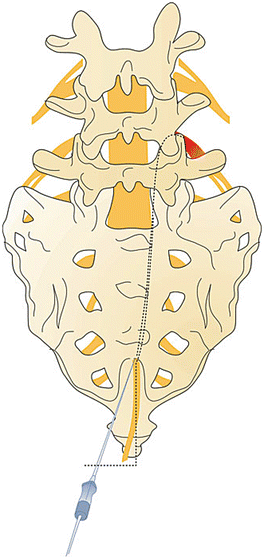
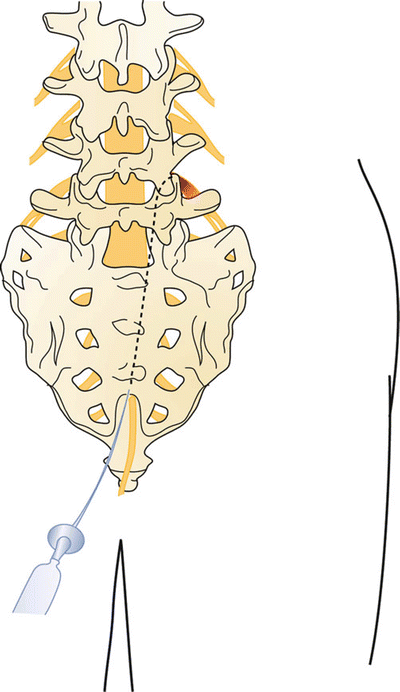
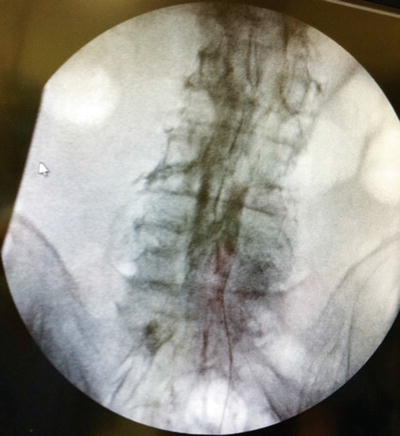

Fig. 31.6
The needle is placed through the sacral hiatus into the sacral canal and rotated in the direction of the target. Do not advance beyond the S3 foramen

Fig. 31.7
The Epimed Racz catheter is marked for the location of the bend, or use the thumb as reference for the 15° angle bend

Fig. 31.8
The direction of the catheter is just near the midline, direct the curve under continuous fluoroscopic guidance to the ventral-lateral target site. The needle rotation, as well as the catheter navigation, may need to be used to reach the target

Fig. 31.9
The needle is removed, and the catheter is placed in the ventral-lateral epidural space ventral to the nerve root

Fig. 31.10
Catheter (24 × L) is threaded to right lateral L4 neural foramen
Once at the target, inject 2–3 mL of additional contrast through the catheter under real-time fluoroscopy in an attempt to outline the “scarred in” nerve root. If vascular uptake is noted, reposition the catheter and reinject contrast. Preferably, there should not be vascular runoff, but infrequently secondary to venous congestion, an epidural pattern is seen with a small amount of vascular spread. This is acceptable as long as the vascular uptake is venous in nature and not arterial. Extra caution should be taken when injecting the local anesthetic to prevent local anesthetic toxicity. Any arterial spread of contrast always warrants repositioning of the catheter. We have never observed intra-arterial placement in over 25 years of placing soft spring-tipped catheters.
Inject 1,500 U of hyaluronidase dissolved in 10 mL of preservative-free normal saline. A newer development is the use of Hylenex or human-recombinant hyaluronidase, which carries the advantage of a reportedly increased effectiveness at the body’s normal pH compared to bovine-recombinant hyaluronidase [20




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree