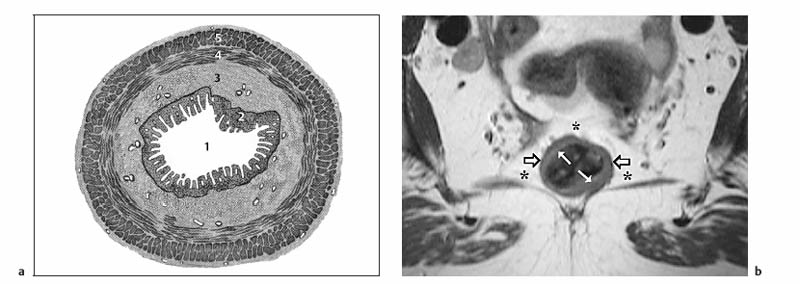
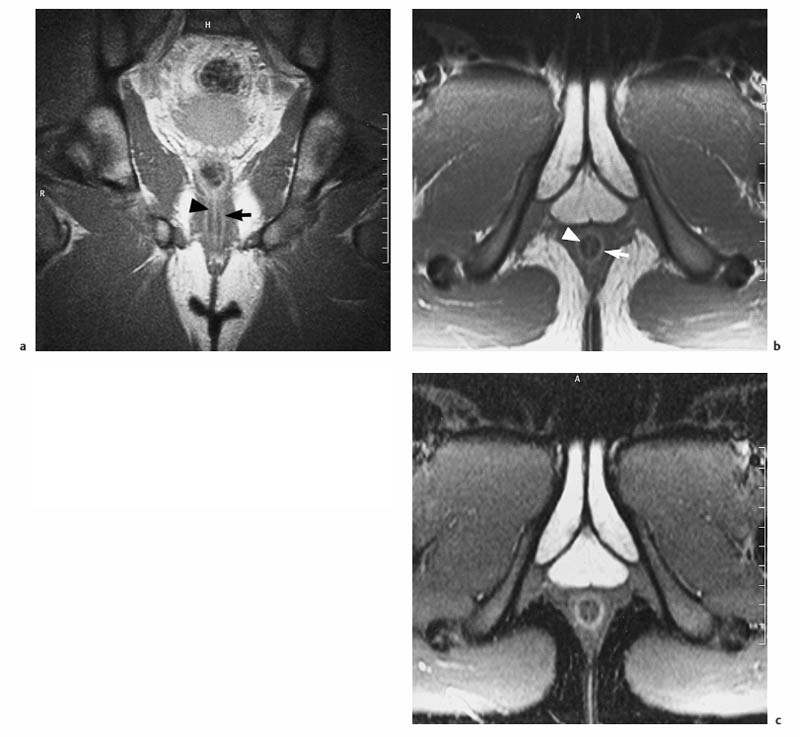
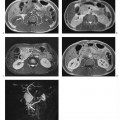
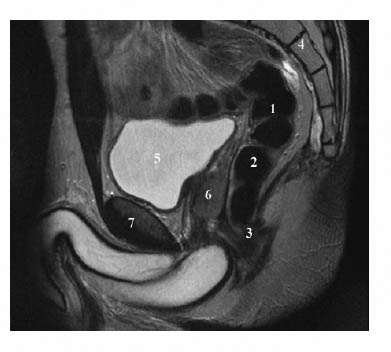
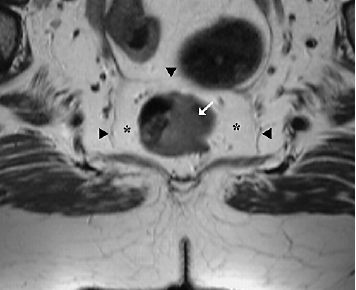
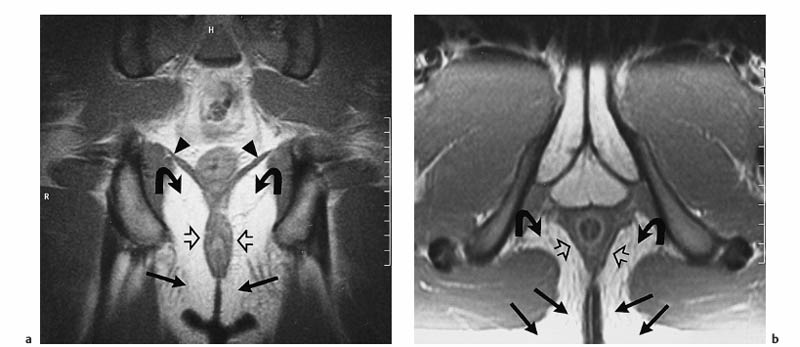
6 The Rectum and Anal Canal With the advent of powerful gradients and high-resolution surface and endorectal coil systems, MRI is increasingly used to evaluate inflammatory and neoplastic diseases of the rectum and anal canal. MRI is superior to endoscopic and endosonographic techniques in that it enables noninvasive evaluation of the rectal lumen and wall and additionally provides detailed information on surrounding structures in the true pelvis. Accurate information on the extent of a disease process and possible involvement of adjacent structures is crucial for tailoring surgical or medical treatment to the individual patient’s needs. The superior tissue contrast provided by MRI is an advantage over CT, but ongoing technical developments may redefine the role of CT in assessing the local extent of rectal tumors. Evaluation for nodal and distant metastases in patients with colorectal cancer is still the domain of CT. Currently, MRI has the following indications in the diagnostic assessment of the rectum, anal canal, and pelvic floor: Before the examination, a careful explanation of the procedure is given, and patients are informed about the risks of administration of an antispasmodic agent and asked about possible contraindications. A venous cannula for contrast injection is placed before image acquisition in those cases where additional contrast-enhanced pulse sequences need to be acquired. Unless contraindicated, an antispasmodic agent (butylscopolamine or glucagon) should be given to reduce artifacts caused by bowel motion. Antispasmodics have very short half-lives and an intravenous injection should therefore be given immediately before the start of image acquisition. The patient should be in a comfortable supine position, with the knees supported by a foam pad if necessary. Before the examination, the patient is instructed to lie still during acquisitions. Most MRI examinations of the rectum can be performed without giving an enteral contrast medium. Some authors advocate the use of a positive or negative enteral contrast agent, but there appears to be general agreement in the current literature that this is not necessary. No intraluminal contrast is needed for MRI in patients with inflammatory rectal disease. Some clinical indications require intravenous injection of an extracellular, Gd-based contrast medium such as Magnevist (Gd-DTPA) or Dotarem (Gd-DOTA). The standard dose is 0.1 mmol Gd per kg body weight, and the contrast medium is automatically injected during the examination. Ideally, phased-array surface coils should be used because they provide a much better signal-to-noise ratio (SNR).1 The coils are positioned according to the target region and kept in place with belts. When first introduced, endorectal coils appeared promising, but they have failed to establish themselves for imaging of the rectum and anal canal because they are cumbersome to handle, expensive (disposable systems), and have a limited field of view (FOV). Following the usual three-plane localizer, sequences in the sagittal and axial planes are best suited for imaging the rectum and anal canal and evaluating their relationship to surrounding structures in the true pelvis. When MRI is performed for suspected fistula, additional coronal images should be obtained, and sagittal images may not be needed (depending on the site of the fistula). In patients with rectal cancer, imaging in an oblique axial plane perpendicular to the lumen of the affected bowel segment is necessary to evaluate the wall and identify invasion into perirectal fat. Additional coronal images will improve identification of possible involvement of the anal sphincter in patients with low rectal cancer. After a three-plane localizer, fast breath-hold sequences in at least two planes (e. g., T2w HASTE) should be obtained to define the exact area involved and plan the subsequent sequences. These sequences have extremely short acquisition times and are fairly insensitive to motion artifacts. In patients with inflammatory bowel disease, an inversion recovery sequence with a short inversion time (e. g., TIRM, STIR) is acquired next for detection of fluid in abscess cavities and fistula tracts, making such a sequence especially useful when looking for perianal and perirectal fistulas. Unenhanced images for assessment of local tumor extent in patients with rectal cancer are obtained using high-resolution T2w TSE sequences, which provide a better contrast-to-noise ratio (CNR) and markedly higher spatial resolution than T2w HASTE sequences, but acquisition times are much longer and they are more susceptible to motion artifacts. It is especially with these sequences that imaging quality can be markedly improved by placement of saturation bands over the anterior subcutaneous fat and administration of an antispasmodic agent (see above). In patients with rectal inflammatory disease, intravenous contrast medium will improve evaluation of disease activity and delineation of anorectal fistula tracts and abscesses. Though we present some examples of contrast-enhanced images, investigators seem to agree that no intravenous contrast medium is needed for initial imaging of rectal cancer.2 Intravenous contrast may be helpful, though, in patients with suspected tumor recurrence. Contrast-enhanced MRI is performed using T1w SE or TSE sequences with spectral fat saturation. The pelvic lymph nodes are best imaged with axial T1w or PD sequences, covering the entire area from the aortic bifurcation to the pelvic floor (see Chapter 16). Parallel imaging techniques (SENSE, iPAT) can be used to shorten acquisition time,3 but this is accomplished at the expense of SNR. Several recent studies suggest that certain diffusion and perfusion parameters may predict the response to radiochemotherapy, which is why the use of diffusion and perfusion-weighted imaging (DWI and PWI sequences) is under clinical investigation.4–6 Details of the recommended pulse sequences and imaging parameters are summarized in Tables 6.1 and 6.2. The rectum is ca. 12–15 cm in length and extends from the level of the third sacral vertebra, where the mesentery of the sigmoid colon ends, to the anus (Fig. 6.1). Unlike the colon, the rectum has no teniae, haustra, or omental appendices. When viewed laterally, the rectum has two bends—the upper sacral flexure with posterior convexity and the lower anorectal flexure with anterior convexity. The rectum can be divided into the pelvic rectum or rectal ampulla, a contractile reservoir, and the perineal rectum or anal canal. The latter is ca. 3 cm long and extends from the levator ani muscle to the anus. Surgeons typically refer to the anal canal as the portion of the rectum below the pectinate or dentate (anorectal) line, which marks the transition from the columnar epithelium of the rectal mucosa to the squamous epithelium of the anoderm. Fistulas in this area are therefore designated as perianal fistulas in the radiologist’s report. A second line, the anocutaneous line, demarcates the transition from the hairless skin of the anal canal to the perianal skin. The normal wall layers of the rectum are depicted diagrammatically in Fig. 6.2a. The anterior and lateral surfaces of the upper two thirds of the ampulla are covered by peritoneum. The part of the rectum above the middle transverse rectal fold (Kohlrausch fold) is retroperitoneal, while the portion below is extraperitoneal. The rectal fascia constitutes a cylindrical sheath surrounding the rectum down to the pelvic floor and is referred to as the mesorectal fascia in the clinical and surgical literature7 (Fig. 6.3). Posteriorly, the mesorectal fascia is thickened and overlies the parietal pelvic fascia, which covers the sacrum. So-called total mesorectal excision (TME) of rectal cancer is the en bloc resection of the tumor-bearing rectum within its enveloping fascia including lymphatics, lymph nodes, and mesorectal fat, while preserving the parietal pelvic fascia and pelvic splanchnic nerves (nervi erigentes). Closure of the anal canal is ensured by the external and internal anal sphincters and levator ani muscle in the wall of the anal canal. Muscular closure is supported by the anal cushions (corpora cavernosa recti) (Figs. 6.4 and 6.5). The rectum above the anal canal is supplied by the superior rectal artery, which is the continuation of the inferior mesenteric artery. The paired middle rectal arteries arising from the internal iliac or internal pudendal artery course laterally through the paraproctium to supply the anal canal and lower portion of the ampulla. At least three layers of the rectal wall can be distinguished on high-resolution T2w MR images: an inner layer of high signal intensity comprising the mucosa and submucosa; a middle layer of low signal intensity, which corresponds to the muscularis propria; and an outer layer of high signal intensity representing the perirectal fat (Fig. 6.2b). Under optimal conditions, with the bowel relaxed and empty, the mucosa is seen as a thin line of low signal intensity. The mesorectal fascia is consistently depicted as a thin, hypointense line surrounding the rectum and perirectal fatty tissue. On contrast-enhanced T1w images, the mucosa and muscularis mucosae enhance early and intensely, differentiating these two layers from the nonenhancing muscularis propria. Perirectal fat has high signal intensity on non-fat-suppressed T1w images (Fig. 6.5). Fig. 6.1 Normal MR appearance of the rectum in a male on a sagittal T2w TSE image. Sacral flexure (1) anorectal flexure (2), anal canal (3), sacrum (4), urinary bladder (5), prostate (6), and pubic symphysis (7). Fig. 6.3 Normal appearance on axial T2w TSE image. Note good delineation of the mesorectum (*) and mesorectal fascia (arrowheads). Stool is present in the rectum (arrow). Fig. 6.4a, b Normal anatomy. a Coronal TSE image. b Axial PD TSE image. Curved arrows indicate the ischiorectal fossa, open arrows the para-anal space, and straight arrows the subcutaneous space. The coronal image (a) clearly depicts the levator ani muscle on both sides (arrowheads). Colorectal cancer is the third most common cancer worldwide. In the United States, ca. 145 000 new cases and 56000 deaths were estimated for 2005.8
C. Klessen and M. Laniado
Introduction
Indications
Imaging Technique
Patient Preparation and Positioning
Contrast Media
Coils
Imaging Planes
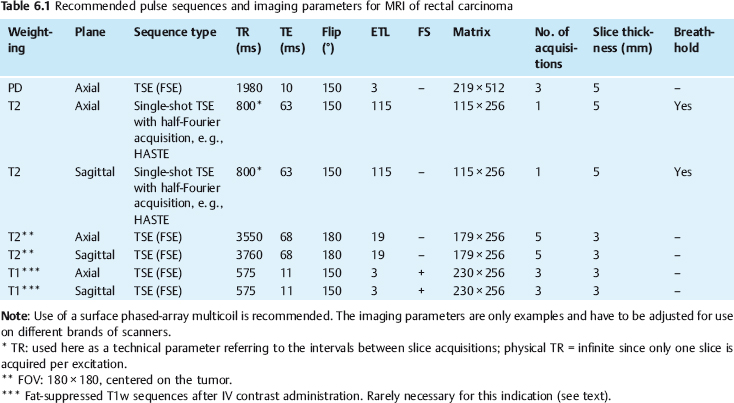
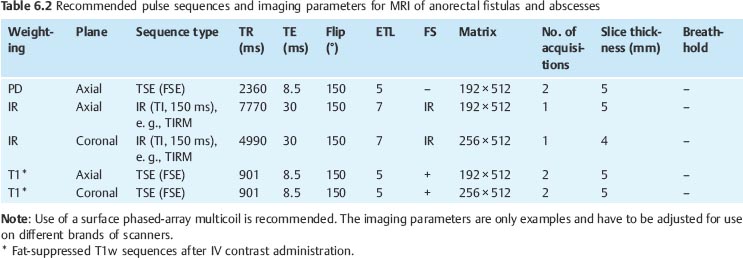
Imaging Protocol
MRI Appearance of Normal Anatomy
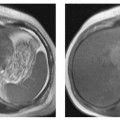
MRI Appearance of Pathologic Entities
Rectal Carcinoma
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree