The Retroperitoneum
The several spaces and potential spaces that compose the retroperitoneum are discussed in the chapter on anatomy. The subject of this chapter is those conditions, which involve the urinary tract such as fluid collections, retroperitoneal fibrosis, lymphadenopathy, and primary retroperitoneal tumors.
▪ FLUID COLLECTIONS
Hemorrhage
The most common nontraumatic cause of retroperitoneal hemorrhage is rupture of an abdominal aortic aneurysm. Other sources of bleeding include diseases of the kidney or adrenal gland. Retroperitoneal hemorrhage can be massive and life threatening, often without significant symptomatology until late in its course. Therefore, a high index of suspicion is necessary to enable diagnosis of the condition and the source of bleeding.
Abdominal aortic aneurysm, defined as an aortic diameter greater than 3 cm, may result in catastrophic hemorrhage. When less than 5 cm in diameter, an aneurysm has approximately a 5% chance of rupturing, whereas the risk increases to greater than 75% with aneurysms greater than 7 cm in diameter. Aneurysmal dilation involves all layers of the aortic wall, including the intima, media, and adventitia. A pseudoaneurysm, on the other hand, results from a prior perforation of the aorta with subsequent resolution of the hematoma and formation of a cavity that is lined by adventitia only. Men are diagnosed with abdominal aortic aneurysms far more commonly than are women. Most aneurysms are atherosclerotic in origin, but on rare occasions can be secondary to prior trauma or mycotic infection. They may also occur in association with Marfan syndrome. On occasion, an inflammatory cuff of tissue (perianeurysmal fibrosis) can surround the aneurysm and may be difficult to differentiate from leakage of blood. Perianeurysmal fibrosis can envelop and obstruct one or both ureters and is considered a variant of retroperitoneal fibrosis.
Several imaging techniques are useful in evaluating aortic aneurysms. Wall calcifications can be seen on plain films of the abdomen in up to 85% of cases. Sonography is often used both for screening patients for suspected aneurysms and for follow-up of aneurysmal growth when initial treatment is conservative. The aorta is seen as a tubular, echofree structure in the left prevertebral area in both longitudinal and transverse views. Moderately echogenic soft tissue within the lumen is an indicator of intraluminal thrombus. The inability of sonography to consistently demonstrate the cephalad extent of an aneurysm in relation to the renal arteries is its major weakness.
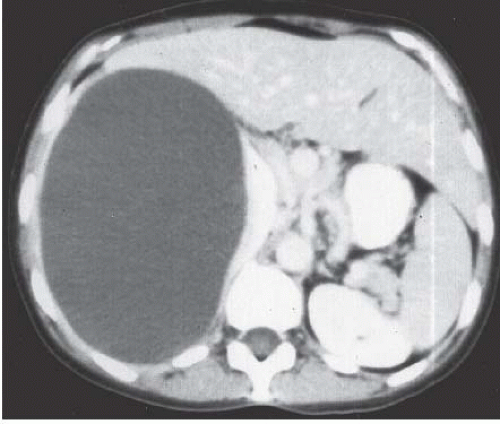
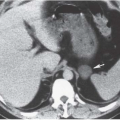
Both computed tomography (CT) and magnetic resonance imaging (MRI) are highly capable modalities for evaluating aortic aneurysms (Fig. 5.1). This evaluation should include the craniocaudad extent of the lesion, involvement of the renal arteries, or extension into the common iliac arteries, maximum diameter of the aneurysm, the presence or absence of intraluminal thrombus, and any indication of leakage (Fig. 5.2).
Following aortic aneurysm, the most common cause of retroperitoneal hemorrhage is disease of the adrenal glands or kidneys. Although the term spontaneous implies the lack of observable injury, in many instances, it is likely that the patient has had mild unrecognized trauma.
Both primary and metastatic adrenal tumors can hemorrhage spontaneously. Other adrenal lesions that may bleed include myelolipomas, pheochromocytomas, cysts, and abscesses. In patients with severe stress, hemorrhagic diathesis, or coagulopathy, the adrenal hemorrhage may occur without a predisposing lesion.
In the kidneys, renal neoplasm and renal artery aneurysm are the most common causes of unprovoked bleeding. Although bleeding from virtually any renal neoplasm may occur, hemorrhage usually is from renal cell carcinoma or angiomyolipoma. Although angiomyolipomas tend to be greater than 4 cm at the time they hemorrhage, renal cell carcinomas may be quite small, and often peripherally located. In these latter cases, detection of the primary
lesion can be difficult. If no etiology for the hemorrhage is seen on an enhanced CT examination of the kidneys, the examination should be repeated after the hematoma has resorbed in order to avoid missing a renal tumor.
lesion can be difficult. If no etiology for the hemorrhage is seen on an enhanced CT examination of the kidneys, the examination should be repeated after the hematoma has resorbed in order to avoid missing a renal tumor.
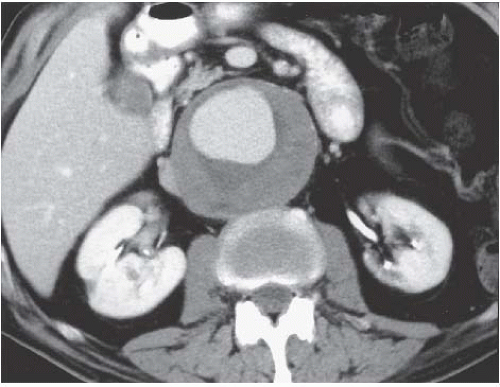 FIGURE 5.1. Abdominal aortic aneurysm. An 8-cm aneurysm is demonstrated clearly on this enhanced abdominal CT examination. |
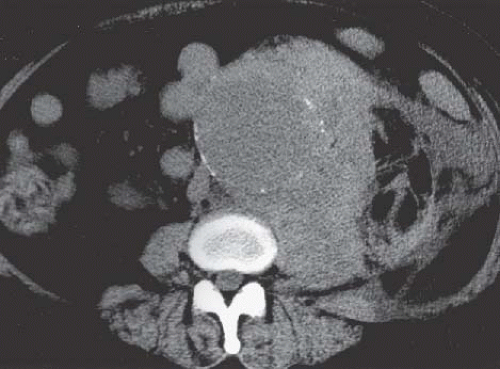 FIGURE 5.2. Hemorrhage from aortic aneurysm. High-density blood is seen adjacent to a large abdominal aortic aneurysm. |
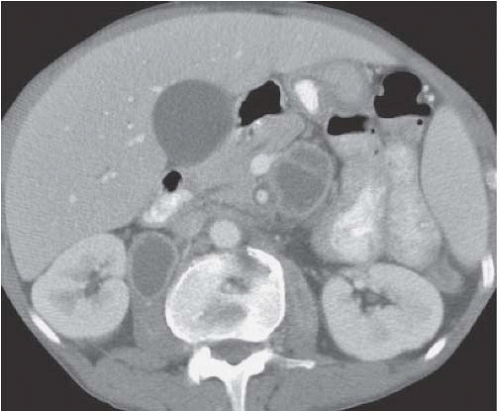
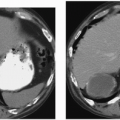
Multiple other types of renal lesions can hemorrhage spontaneously, including polyarteritis nodosa, inflammatory lesions, arteriovenous malformation, hydronephrosis (congenital ureteropelvic junction obstruction), and rupture of a renal cyst or even a vessel in the wall of a simple cyst (Fig. 5.3). In some cases, retroperitoneal hemorrhage may be idiopathic. The reported incidence where no etiology can be found ranges from 4% to 15%.
Most instances of retroperitoneal hemorrhage, whether from an aortic aneurysm or a lesion of the kidney or adrenal gland, occur into the perinephric space. Hemorrhage confined to the anterior pararenal space can be from hemorrhagic pancreatitis or trauma to the duodenum or pancreas. Isolated hemorrhage into the posterior pararenal space, which contains only fat and vessels, usually occurs in patients on anticoagulant therapy (Fig. 5.4). Subcapsular hematomas can be distinguished from perinephric hematomas by the compressive effect they exert on the renal parenchyma (Fig. 5.5).
CT is the modality of choice for evaluating patients with a suspected retroperitoneal bleed. Not only is CT highly sensitive in detecting the hemorrhage, but also it provides a clear delineation
of the extent and location of the hemorrhage. Acute hemorrhage is seen as an accumulation of fluid with an attenuation measuring 40 to 60 Hounsfield units (HU). If the hematoma is stable and treated conservatively, follow-up CT will show a decreasing attenuation of the hematoma over time, approaching water density during the final stages of resolution. When studied by MRI, acute hematomas generally exhibit low to medium signal intensity on T1-weighted images, becoming brighter on T2-weighted images. As the hematoma ages, the periphery increases in signal intensity on T1-weighted images, and in the chronic phase there is a uniform high intensity on both T1 and T2 images.
of the extent and location of the hemorrhage. Acute hemorrhage is seen as an accumulation of fluid with an attenuation measuring 40 to 60 Hounsfield units (HU). If the hematoma is stable and treated conservatively, follow-up CT will show a decreasing attenuation of the hematoma over time, approaching water density during the final stages of resolution. When studied by MRI, acute hematomas generally exhibit low to medium signal intensity on T1-weighted images, becoming brighter on T2-weighted images. As the hematoma ages, the periphery increases in signal intensity on T1-weighted images, and in the chronic phase there is a uniform high intensity on both T1 and T2 images.
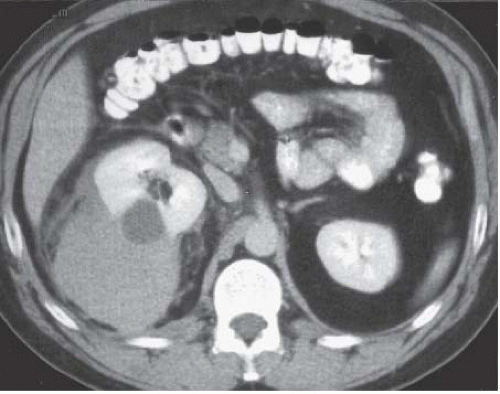 FIGURE 5.3. Perinephric hematoma. A large, high-density fluid collection extends from the right kidney into the perinephric space. |
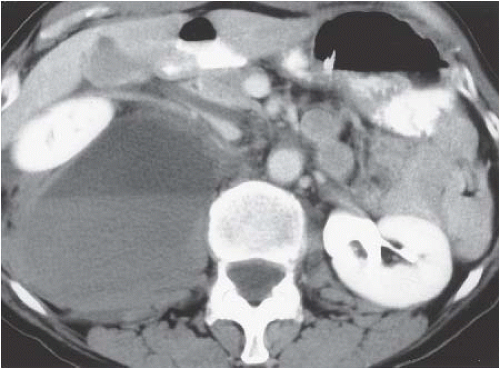 FIGURE 5.4. Posterior pararenal hematoma. Hemorrhage in the posterior pararenal space is causing anterior displacement of the right kidney. |
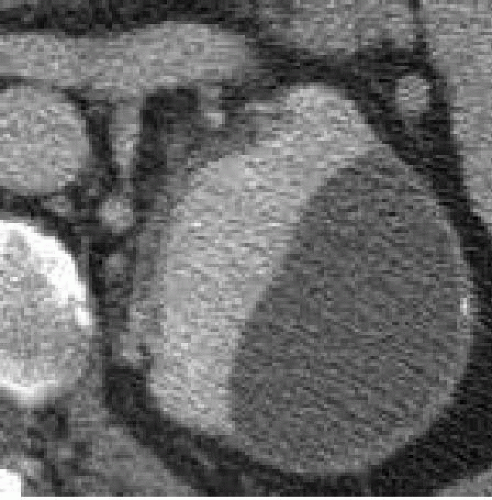 FIGURE 5.5. Subcapsular hematoma. The flattened surface of the kidney indicates this is a subcapsular fluid collection. |
Urinoma
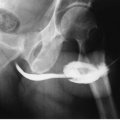
A urinoma is a mass formed by encapsulated extravasated urine. It may be subcapsular in location (Fig. 5.6), confined to the perirenal space (extracapsular), or may transcend fascial boundaries and track into the peritoneal cavity (urinary ascites). Urinomas most typically occur following renal injury and, when subcapsular, may compress the renal parenchyma, activate the renin system, and result in significant hypertension, a condition known as Page kidney.
Other etiologies of urine extravasation include surgery and spontaneous extravasation of urine secondary to obstruction. Postoperative urine leak usually is recognized and resolves with appropriate drainage or stenting until healing is complete. Pyelosinus extravasation of urine associated with obstruction also usually resolves with treatment of the obstructive process. In these cases, urine leaks from a calyceal fornix into the renal sinus, and tracks along the renal hilum and ureter into the lower cone of the perinephric space. Leakage of uninfected urine is seldom of concern, and occasionally is seen in acute obstruction secondary to ureteral calculi. If infected, extravasated urine may lead to abscess formation and should be treated with antibiotics.
Clinical features of urinoma, aside from hypertension in the rare case of Page kidney, include malaise, vague abdominal pain, weight loss, and a palpable mass. When untreated, the extravasated urine stimulates an intense fibrous reaction that results in a thick-walled collection. Alternatively, urine in the retroperitoneum may result in retroperitoneal fibrosis as opposed to the more typical encapsulated collection. Therefore, early diagnosis and treatment are essential.
As with hemorrhage, urine collections typically confine themselves to the various compartments and can be localized with crosssectional imaging techniques such as CT and MRI. Uninfected urinomas demonstrate water attenuation on CT and the signal characteristics of water on MRI. With an acute leak of urine from the collecting system or bladder, contrast studies such as CT, excretory urography (IVP), or cystography may demonstrate contrast in the urine collection that clearly is outside the confines of the urinary tract.
Abscess
Nonspecific symptoms such as nausea, vomiting, weight loss, fever, and anorexia may herald the onset of a retroperitoneal abscess. On occasion, there may be pain localized to the area of the abscess, or if the abscess is adjacent to or involves the psoas muscle (Fig. 5.7), the patient may complain of pain referred to the ipsilateral hip that is exacerbated by extension or rotation of the thigh.
More than 50% of retroperitoneal abscesses occur in the anterior pararenal space and are associated with pancreatitis. Those in the perirenal space almost always are associated with a urinary tract infection. Osteomyelitis of the adjacent spine or ribs is the most likely cause of an abscess in the posterior pararenal space.
Radiographically, an abscess is seen as a thick-walled fluid collection. Internal echoes or an attenuation greater than water are a result of cellular debris within the abscess. Gas is seen in only a minority of abscesses but suggests infection when present. Inflammatory changes often are seen adjacent to the abscess.
As with retroperitoneal hemorrhage, CT is an ideal modality for detecting retroperitoneal abscesses and defining their extent. The purulent fluid in an abscess usually exhibits an attenuation slightly higher than water. Once the diagnosis has been made, CT or ultrasonography (US) may be used for guiding diagnostic needle aspiration and percutaneous placement of a drainage catheter.
When studied by MRI, the signal intensity of abscess fluid is variable and dependent on the protein content of the fluid as well as on the amount of debris present. On T1-weighted images, purulent fluid generally has a low-signal intensity, becoming bright on T2-weighted images.
Lymphocele
Typically occurring as a complication of pelvic or retroperitoneal surgery (lymphadenectomy or renal transplantation), lymphoceles are discrete collections of chylous fluid. They usually occur because of inadequate ligation of disrupted lymphatic vessels. Typically presenting
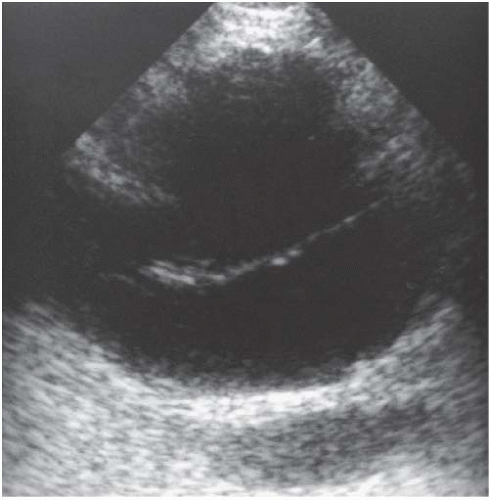
several weeks after surgery, they have been reported as occurring from as early as several days to as late as years after surgery. When quite large, lymphoceles may cause venous obstruction and resultant edema of the lower body. Further, they may result in painful abdominal distention and obstruction of the ureters. When imaged, lymphoceles may be difficult to distinguish from other retroperitoneal fluid collections.
several weeks after surgery, they have been reported as occurring from as early as several days to as late as years after surgery. When quite large, lymphoceles may cause venous obstruction and resultant edema of the lower body. Further, they may result in painful abdominal distention and obstruction of the ureters. When imaged, lymphoceles may be difficult to distinguish from other retroperitoneal fluid collections.
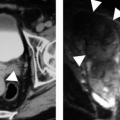
Ultrasound evaluation of a lymphocele typically reveals a fluid-filled mass with absent or faint echoes, though significant septations (Fig. 5.8) and dependent debris may be present. Unfortunately, these findings may also occur in an abscess or resolving hematoma, but the clinical history should suggest the correct diagnosis. CT may be more definitive, with negative attenuation values of the fluid collection suggesting the fatty composition of chyle. Large lymphoceles can be treated using percutaneous catheter drainage, although recurrence is common. Washing the cavity with a sclerosing agent may stimulate fibrosis of the lymphocele and decrease the incidence of recurrence. Small lymphoceles may respond to simple needle aspiration or spontaneously resolve.
Pelvic Fluid Collections
Fluid collections in the pelvis may be intraperitoneal or may lie in the prevesical extraperitoneal compartment. The prevesical space is directly contiguous with the retroperitoneal compartment below the cone of renal fascia. Fluid in the prevesical extraperitoneal space typically has a “molar tooth” configuration on CT or axial MRI images. The “crown” portion of the “tooth” lies anterior to the urinary bladder, whereas the “roots” of the tooth extend posteriorly on either side of the bladder. These roots are frequently asymmetric, resulting in bladder displacement away from the midline when the extraperitoneal pelvic fluid collections are large.
▪ RETROPERITONEAL FIBROSIS
Retroperitoneal fibrosis is a proliferation of fibrotic tissue in the retroperitoneum. Most cases are idiopathic, but the frequent association with thyroiditis, mediastinitis, and sclerosing cholangitis suggests that it is part of a systemic condition. Benign etiologies include an adverse response to various pharmaceutical agents, previous radiation therapy or surgery, and the inflammatory effect of retroperitoneal fluid collections. Various neoplasms can metastasize to the retroperitoneum and incite a desmoplastic fibrotic response. Tumors associated with retroperitoneal fibrosis include Hodgkin disease and other lymphomas, certain sarcomas, and anaplastic carcinomas of the breast, stomach, colon, prostate, kidney, and lung. Grossly and radiographically, the findings are often identical in both benign and malignant retroperitoneal fibrosis; though a lobulated contour, illdefined borders of the plaque, or displacement of the great vessels away from the spine suggests a malignant process (Fig. 5.9).
CAUSES OF RETROPERITONEAL FIBROSIS INCLUDE | |
▪ | Idiopathic (Ormand disease) |
▪ | Malignancy |
▪ | Methysergide therapy |
▪ | Radiation therapy |
▪ | Retroperitoneal bleeding |
▪ | Abdominal aortic aneurysm (perianeurysmal fibrosis) |
Retroperitoneal fibrosis can be found anywhere from the neck to the deep pelvis, but is most frequently localized in the region of the lower abdominal aorta and common iliac arteries. Grossly, retroperitoneal fibrosis is seen as a gray-white, reasonably welldelineated plaque of fibrous tissue with an unyielding consistency. This plaque can extend laterally beyond the confines of the psoas muscles and entrap the ureters.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree