Cardiac magnetic resonance (CMR) imaging has gained significant traction as an imaging modality of choice in the evaluation of individuals with, or at risk for, heart failure. Ventricular arrhythmias, often malignant, may be sequelae of heart failure and arise from fibrosis. Late gadolinium enhancement evaluation by CMR has become a preferred modality to assess individuals at risk for malignant ventricular arrhythmias. A spectrum of various pathologies that predispose individuals to malignant ventricular arrhythmias, as well as the usefulness of CMR in their identification and prognostication, are reviewed.
Key points
- •
Myocardial fibrosis often results in tissue heterogeneity resulting in re-entry circuits and thereby predisposing individuals to malignant ventricular arrhythmias and sudden cardiac death.
- •
Cardiac magnetic resonance (CMR) imaging can provide valuable information to facilitate the management of patients presenting with malignant ventricular arrhythmias of ischemic and nonischemic origins.
- •
By virtue of its strength in tissue characterization sequences such as late gadolinium enhancement imaging, CMR can provide additional information in a myriad of diseases in relation to prognostication and risk stratification.
Introduction
Ventricular arrhythmias are common in patients with cardiomyopathy and heart failure of ischemic and nonischemic origin. These include premature ventricular contractions (PVC), nonsustained ventricular tachycardia (NSVT), accelerated idioventricular rhythm, sustained ventricular tachycardia (VT), and ventricular fibrillation (VF). PVCs are frequently seen in individuals with heart failure, often in up to 70% to 95% of such individuals. They may vary in frequency (such as ventricular bigeminy and trigeminy) or complexity (multifocal or couplets). Complex PVCs may predict more malignant arrhythmias and sudden cardiac death (SCD). PVCs in individuals with previous myocardial infarctions (MIs) are associated with increased risk of death. Malignant ventricular arrhythmias include sustained VT, torsades de pointes (a form of polymorphic VT), and VF. Patients with spontaneous, sustained VT or VF have a much higher risk of SCD.
Cardiac fibroblasts comprise approximately 90% of the noncardiomyocyte cells of the heart and are the primary component responsible for many of the extracellular matrix components, including collagens I, III, IV, laminin, and fibronectin. Histologically, a signature feature of worsening heart failure is progressive accumulation of collagen in the heart, resulting in myocardial fibrosis. This scarring or replacement fibrosis is a compensatory mechanism of the heart to substitute for normal parenchyma. Myocardial fibrosis and the resultant heterogeneity often provide the structural substrate for malignant ventricular arrhythmias, which often culminate in SCD.
Cardiac magnetic resonance (CMR) imaging is an advanced, multifaceted noninvasive imaging modality that can accurately assess myocardial structure and physiology. Specifically, the usefulness of gadolinium-based contrast and late gadolinium enhancement (LGE) makes it a powerful tool to assess the presence of scar, as it disperses in the extracellular space, with excellent histologic correlation. Gadolinium is injected intravenously and can diffuse across the intravascular space but does not cross an intact myocardial cell membrane. Areas of myocardial injury, however, are associated with higher distribution/unit volume, and appear bright on LGE images. The distribution of LGE assists in determination of the various causes of cardiomyopathies, and serves as a prognostic marker for SCD.
Compared with other imaging modalities, CMR has a distinct advantage of its high spatial resolution, allowing for clear scar delineation and quantification, which has prognostic value in determination of SCD. In addition, CMR is not limited by poor acoustic windows and provides more accurate detection of left ventricular ejection fraction (LVEF) compared with transthoracic echocardiography, which is seminal in further management as primary prevention of individuals with malignant arrhythmias and the decision of implantable cardiac defibrillators (ICDs) implantation relies on this.
Cardiac conduction system and introduction to cardiac magnetic resonance imaging
Coordinated contraction of the heart relies on the cardiac conduction system, which consists of specialized cardiomyocytes that generate and propagate electrical impulse. In a normal heart, electrical impulses arise from the sinoatrial junction, which is located near the superior cavoatrial junction. The impulse then travels to and is delayed at the atrioventricular node, near the interatrial septum. This delay allows for atrial contraction and ventricular filling before ventricular contraction. Electrical passage from the atrium to the ventricular myocardium is through the atrioventricular bundle (of His). This bundle conducts the electrical impulse to the left and right bundles and ultimately to the Purkinje fiber network, which activates the ventricular myocardium. Injury or pathology along this pathway can lead to arrhythmias.
A basic CMR imaging protocol for the investigation of ventricular arrhythmias requires assessment of cardiac anatomy and function. Functional assessment relies on cine cardiac imaging through the different cardiac planes (short and long axes), which typically uses steady-state free precession (SSFP) sequences. SSFP sequences are modified gradient echo sequences, which provide excellent contrast between the bright blood and the myocardium with high temporal resolution, making it indispensable in the evaluation of wall motion and in volumetric measurement necessary in the assessment of ventricular function. Detection of myocardial pathology relies on late gadolinium-enhanced mages following intravenous contrast administration and myocardial nulling. Myocardial nulling is achieved although inversion recovery pulses, which result in a dark appearance of the normal myocardium. Myocardial fibrosis or scarring is characterized by LGE, and the pattern and extent of LGE may be indicative of specific diagnoses. Depending on clinical suspicion, additional sequences may be added to the protocol to answer specific diagnostic questions. These sequences are discussed as they pertain to specific pathologies below.
Imaging findings/pathology
Ischemic
- •
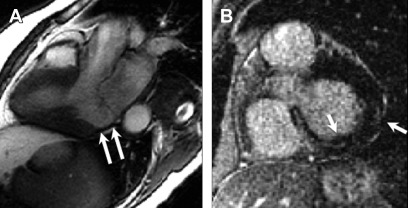
Ischemic heart disease is often associated with LGE, typically in a subendocardial or transmural distribution in myocardial segments, following a coronary artery territory ( Fig. 1 ).
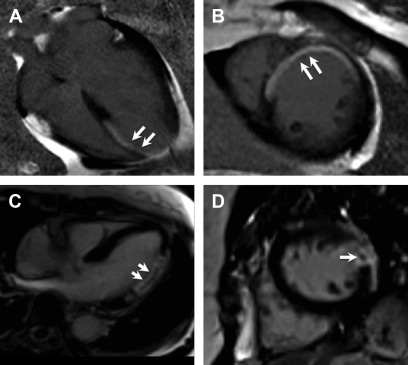
Fig. 1
Ischemic cardiomyopathy. Typical subendocardial LGE in the left anterior descending territory including the septal and anterior walls ( arrows , A , B ). Involvement of greater than 50% of the wall thickness portends low likelihood of recovery after revascularization. Transmural LGE of the lateral wall ( C , D ) seen on a different patient found to have spontaneous coronary arterial dissection and occlusion of the first and second obtuse marginal branches. Central focus of nonenhancement ( arrows , C , D ) is suggestive of microvascular obstruction, which is a poor prognostic indicator.
- •
Often, the pattern involves a central core of dense fibrosis within a heterogenous peri-infarct (gray) zone, suggestive of the presence of viable and nonviable myocardium. The extent of LGE is a reliable marker for the likelihood of functional recovery with revascularization, taking a threshold of 50% to determine viability.
- •
Microvascular obstruction (MVO) is seen in a proportion of individuals with acute MI after reperfusion of a previously occluded coronary artery. Typically, it appears as a central dark focus within an area of early enhancing myocardium. This signifies a focal area of absent contrast enhancement within a site of MI. It may be seen on first-pass perfusion, early gadolinium enhancement (EGE) as well as LGE images, but the presence of MVO on LGE is a more accurate prognostic marker than when seen on first-pass perfusion or EGE, in determining risk of adverse left ventricular remodeling and future major adverse cardiovascular events.
- •
The presence and extent of LGE are significant prognostic markers in the setting of coronary artery disease (CAD). According to 1 study, any LGE is associated with a hazard ratio of 10.9 for cardiac mortality.
- •
A group from Duke demonstrated that the extent of total scar (threshold >5% left ventricular [LV] mass) was the most important prognostic parameter in ischemic as well as nonischemic cardiomyopathy.
Nonischemic Cardiomyopathy
Dilated cardiomyopathy
- •
Dilated cardiomyopathy (DCM) is typically associated with no or minimal obstructive CAD. Up to 71% of such patients have been seen to have LGE, often in a midwall distribution, less often in a subepicardial pattern ( Fig. 2 ).
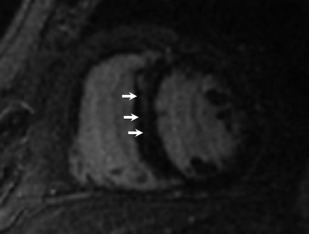
Fig. 2
Dilated cardiomyopathy. Although not specific, linear midwall LGE in the basal septum is commonly seen with dilated cardiomyopathy.
- •
Subendocardial injury is seen in approximately 17% of individuals and may be reflective of embolic phenomena.
- •
The extent of LGE and percentage of LV mass affected are related to the occurrence of inducible VT, SCD, or ICD therapy.
- •
A previous pooled cohort analysis of 7 studies of patients with DCM and ischemic cardiomyopathy demonstrated that, although the presence of scar was an independent predictor of endpoints, the extent of scar (either transmurality or percentage of LV mass) was most predictive of outcomes, including inducible VT on electrophysiology studies, ventricular arrhythmias or ICD therapy, or a composite endpoint of ICD therapy or SCD.
Hypertrophic cardiomyopathy
- •
Hypertrophic cardiomyopathy (HCM) is the most common genetic cardiomyopathy with many genes encoding for cardiac sarcomere. It is characterized by a heterogenic phenotypic expression.
- •
SCD is a catastrophic disease consequence seen in a small subset of patients. It may be unpredictable and often arises in youth or middle-age.
- •
ICD therapy has been instrumental in decreasing rates of SCD in patients with HCM to approximately 0.5%/year.
- •
LGE on CMR has immense potential to identify areas of myocardial fibrosis, which is postulated to be the arrhythmogenic substrate in this cohort, as previous studies have demonstrated that individuals with LGE have higher rates of NSVT on Holter monitoring, compared with those without LGE ( Fig. 3 ).
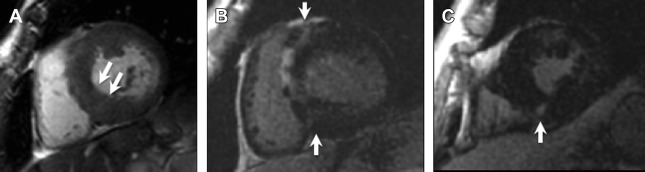
Fig. 3
Hypertrophic cardiomyopathy. Short axis SSFP image ( A ) shows asymmetric septal wall thickening measuring 25 mm in diastole ( arrows , A ). Post-contrast images ( B , C ) show subepicardial and midwall LGE predominantly in the septal and anterior walls with additional nodular LGE at the right ventricular insertion points ( B , C , arrows ).
- •
Extensive LGE, comprising greater than 15% LV mass involvement is associated with a 2-fold increased risk of SCD, regardless of the presence of other risk factors (left ventricular wall thickness >30 mm, family history of SCD, presence of NSVT, unexplained syncope, or abnormal blood pressure response).
- •
The most common locations for LGE involve the hypertrophic segments and the right ventricular (RV) insertion points; however, it may be diffuse and follow a non-territorial pattern.
- •
A threshold of 6SD has been established as a reliable and reproducible metric to determine the extent of LGE in patients with HCM.
Sarcoidosis
- •
Sarcoidosis is a multisystem granulomatous disease of unclear origin with characteristic noncaseating granulomas. Cardiac involvement is clinically evident in about 5% of affected individuals and may result in LV dysfunction, scar, and significant arrhythmias, but autopsy findings demonstrate myocardial lesions in about 20% to 60%. Sarcoidosis is suspected when there is unexplained LV systolic dysfunction with an LVEF less than 40% or if there is unexplained spontaneous or induced sustained VT. The principal manifestations are conduction abnormalities, ventricular arrhythmias including SCD, and heart failure.
- •
Although there is no specific diagnostic pattern of LGE on CMR for cardiac sarcoidosis (CS), it often presents as a patchy or multifocal distribution with sparing of the endocardial border. It is more often seen in the basal segments, especially in the septal and lateral walls, and more often in a midmyocardial to subepicardial distribution. Occasionally, a transmural pattern may also be seen with rare involvement of the RV free wall. Occasionally, one may notice a prominent involvement of the insertion points with direct and contiguous extension across the septum into the RV, and this has been termed the “hook sign.” The presence of this on CMR increases the probability of CS to greater than 90% ( Fig. 4 ).
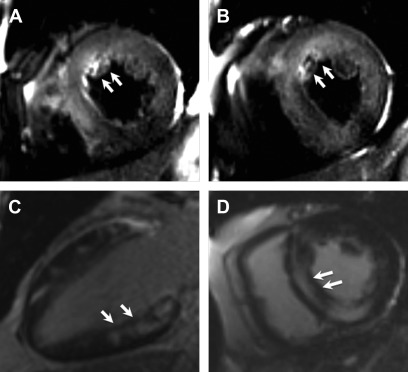
Fig. 4
Cardiac sarcoid. Short-axis T2-weighted image ( A , B ) show near diffuse increased signal particularly of the anterior and septal walls, consistent with myocardial edema ( arrows , A , B ). Two-chamber and short axis post contrast images show patchy and nodular areas of mid-wall myocardial LGE with relative sparing of the subendocardial border ( C , D , arrows ).
- •
A multimodality imaging assessment of 29 patients with CS and VT demonstrated a higher tendency of abnormal electrograms on electroanatomical mapping to correlate with scar on CMR and less so with inflammation on PET, suggesting the dominant role of scar in ventricular arrhythmogenesis.
Myocarditis
- •
The most recent update on the expert consensus recommendations for the CMR diagnosis of acute myocarditis (the “Lake Louse Criteria”) proposes 2 main criteria:
- 1.
The presence of myocardial edema;
- 2.
Evidence of nonischemic myocardial injury.
- 1.
- •
Myocardial edema is indicated by increased myocardial signal on T2-weighted sequences. T2 mapping allows for quantitative assessment of the myocardium, which may increase the accuracy of detecting edema and may have prognostic value.
- •
Myocardial injury is indicated by nonischemic pattern LGE. Classically, although neither sensitive nor specific, LGE in myocarditis has been described to be in subepicardial in the basal-to-mid LV inferolateral segments ( Fig. 5 ).
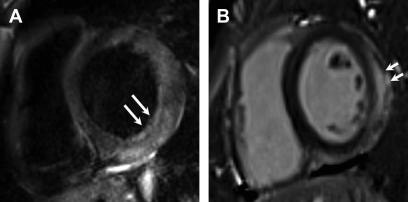
Fig. 5
Acute myocarditis. Short axis dual echo T2-weighted sequence image ( A ) myocardial edema in the basal inferolateral, lateral, and anterolateral segments ( arrows , A ). There is corresponding subepicardial LGE ( arrows , B ) in the same distribution.
- •
Myocardial injury may also be indicated by abnormally increased signal on T1-weighted images or increased extracellular volume, which can be determined using T1 mapping techniques.
- •
Practically speaking, however, in routine clinical practice, the presence of evidence of nonischemic myocardial injury on CMR (ie, nonischemic pattern of LGE) in the appropriate clinical setting would be adequate for the diagnosis of acute myocarditis.
Chagas cardiomyopathy
- •
Chagas disease is an infectious myocarditis caused by the protozoan, Trypanosoma cruzi , which is endemic to Central and South America.
- •
Most patients have a self-limited course, but approximately 30% develop persistent parasitemia and latent infection that manifests years later as a DCM, and subsequently chronic Chagas cardiomyopathy (CCC) may manifest as myocardial damage, characterized by marked fibrosis often associated with ventricular arrhythmias and apical aneurysms.
- •
Scar in patients with CCC has been determined to be a strong predictor of a combination of death and sustained VT.
- •
The distribution of LGE scar is more often seen along the inferolateral wall of the left ventricle, but no specific distribution of fibrosis was noted to correlated with pathogenesis or outcomes. Varying observed LGE patterns included apical, transmural, focal and diffuse ( Fig. 6 ).
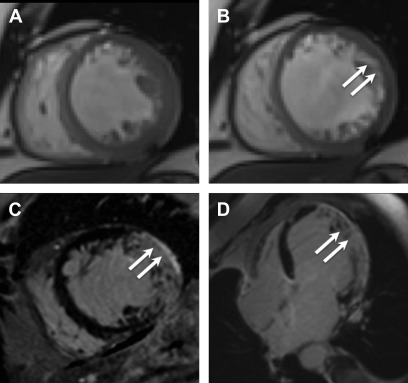
Fig. 6
Chagas cardiomyopathy. Representative still frames demonstrating systolic ( A ) and diastolic ( B ) mid-ventricular short axis slices showing thinning of the lateral wall ( arrows , B ), which was dyskinetic on cine imaging (not shown here). Short axis ( C ) and 4-chamber ( D ) post contrast images show transmural LGE ( arrows , C , D ) in the lateral and inferior walls consistent with acute myocarditis with a differential diagnosis of chronic infarct, subsequently proven to be secondary to Chagas cardiomyopathy based on serology.
Left ventricular noncompaction
- •
Left ventricular noncompaction (LVNC) is an uncommon cardiomyopathy associated with prominent and deep LV trabeculations and intertrabecular recesses, altering the myocardial framework, and thought to be associated with a developmental arrest in the embryogenic period causing lack of compaction of the myocardium.
- •
LGE has been observed in approximately 40% of individuals who meet diagnostic criteria for LVNC. The underlying mechanisms have been postulated to be related to coronary microcirculatory dysfunction, resulting in ischemia and fibrosis in noncompacted and compacted myocardial segments, in a variegated fashion. The distribution has been observed to be heterogeneous as well, and may involve subendocardial, midmyocardial, subepicardial, or transmural segments ( Fig. 7 ). Although some studies have demonstrated a predilection for LGE involvement of the interventricular septum, LGE involvement of the noncompacted segments is often less common. As the presence of LGE in LVNC is infrequent, its presence is specific, but not sensitive enough to make the diagnosis.
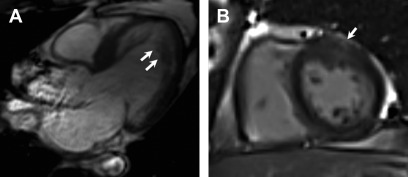
Fig. 7
Left ventricular noncompaction. Three chamber view SSFP image ( A ) shows prominent and deep inter-trabecular recesses with greater than 2.3 ratio of noncompacted to compacted myocardium, particularly of the mid to apical segments ( arrows , A ). There is also patchy subepicardial LGE noted in the anterior wall on post contrast imaging ( B , arrow ).
- •
Hyper-enhancement within the trabeculations of the noncompacted segment may also suggest fibrosis, which may serve as a marker for high-risk patient delineation because this may serve as a focus for malignant ventricular arrhythmias. Another study of 113 patients demonstrated LGE in only 11 patients, but this was predictive of cardiac events, suggestive of a prognostic benefit.
Valvular Diseases
Arrhythmogenic mitral valve prolapse
- •
Similar to findings in echocardiography, mitral valve prolapse (MVP) is characterized by at least 2 mm excursion of the valve leaflet into the left atrium in LV outflow tract views ( Fig. 8 ).

Fig. 8
Arrhythmogenic mitral valve prolapse. Four-chamber SSFP sequence shows ballooning and excursion of the mitral valve leaflet greater than 2 mm into the left atrium ( arrow ).
- •
Presence of LGE in the papillary muscles is suggestive of underlying fibrosis and has been associated with increased risk for complex VTs.
Mitral annular disjunction
- •
On imaging, up to approximately 20% of patients with mitral annular disjunction (MAD) do not have associated MVP, and arrhythmias can be present irrespective of the presence or absence of MVP.
- •
On CMR, MAD is characterized by increased distance of the mitral annular roots to the ventricular myocardium, mainly affecting the posterior leaflet. The finding is best demonstrated in systole, in which there is a typical “curling motion” of the basal posterolateral wall, accentuating the disjunction. Although variable, MAD has been reported to affect up to two-thirds of the mitral annular circumference ( Fig. 9 ).