No invasion
Inner third
Middle third
Outer third
Grade 1
0/55
5/61 (8 %) (2P, 1V)
0/4
Grade 2
0/17
2/41 (5 %)
1/7 (14 %) (1V)
1/2 (50 %) (1V)
Grade 3
1/5 (20 %)
2/7 (29 %) (1V)
1/1 (100 %) (1P)
Similarly, Eifel and associates [20] reported a recurrence rate of 0.8 % (1/127) in patients with non-invasive tumors treated with surgery alone. This recurrence occurred in a patient with an initial grade 1 endometrial carcinoma in whom an anaplastic carcinoma of the pelvic sidewall developed, which the authors believed to be a second primary; it was, however, scored as a recurrence. Price and colleagues [21] also studied the pattern of recurrence in patients with stage I disease treated with surgery alone. They noted a vaginal recurrence rate of 4.4, 5.7, and 13.6 % for well, intermediate, and anaplastic histology, respectively. In the same group, the incidence of recurrence was 3.7 % with no myometrial invasion, 4.7 % with superficial invasion, and 15.1 % with deep myometrial invasion.
Patients with pathologic stage II disease treated with hysterectomy alone are at a higher risk of recurrence than those whose disease is classified as pathologic stage I. The GOG study noted recurrence in seven of 29 patients (four pelvic, one vaginal) treated with surgery alone. Therefore, in this group of patients, the local recurrence rate was approximately 20 % in those who did not receive radiation therapy [17]. In a review by Fanning and coworkers, [22] no patient with stage IIA (based on the old staging system) disease treated with surgery alone had a recurrence compared to five of six patients with stage IIB disease. Other investigators have noted that in patients with stage II disease, histologic grade and depth of invasion remain important prognostic variables [23−25]. Therefore, recurrence rates in patients with stage IIA disease probably are influenced greatly by other known prognostic variables.
Lympho-vascular space invasion has also been noted to be a risk factor for recurrence. Tsuruchi and associates [26] noted a recurrence rate of 30.7 % in clinical stage I and stage II patients with lympho-vascular space invasion vs. 3.2 % in patients who had no invasion. Other authors have noted similar increased recurrence rates [27, 28]. Age is also a prognostic factor for survival. Younger women tend to have a better prognosis than older women. For instance, the GOG reported survival rates of 96.3 % for patients ≤ 50 years old, 87.3 % for patients 51–60 years old, 78 % for patients 61–70 years old, 70.7 % for patents 71–80 years old, and 53.6 % for patients older than 80 [29]. As a general guideline, for every 1-year increase in age, the risk of recurrence increases by 7 % [30]. Patients with stage III disease represent a highly variable group. Patients with extrauterine spread limited to the peritoneal fluid, or adnexa, or both, generally have more favorable outcomes compared to patients with other intra-abdominal metastases. In the GOG study of patients with stage IIIA disease who were treated with surgery alone, the recurrence rate was 0 % (0/2) for adnexal involvement and 7 % (1/14) for positive cytology. This compares with a recurrence rate of 50 % in patients with positive pelvic nodes (stage IIIC) [17].
Lymph node metastasis is the most important prognostic factor in clinical early-stage endometrial cancer. Of patients with cT1 disease, 10 % will have pelvic and 6 % will have para-aortic lymph node metastases [17]. Patients with lymph node metastases have an almost six times higher risk of developing recurrent cancer than patients without lymph node metastases. One study showed a recurrence rate of 48 % for patients with positive lymph nodes, including 45 % with positive pelvic nodes and 64 % with positive aortic nodes, compared to 8 % for patients with negative nodes. The 5-year DFS rate for patients with lymph node metastases was 54 % compared with 90 % for patients without lymph node metastases [30].
Peritoneal relapse accounts for almost 25 % of all recurrences in a study by Mariani et al. that included 599 patients with both stage IV disease and stage I–III disease. Any two of four independent factors (nonendometrioid histology, positive peritoneal cytology, cervical stromal invasion and lymph node metastases) were identified as predictors for peritoneal failure [31]. The recurrence rates for papillary serous histology, even when confined to the uterus, range from 50 to 85 %, with upper abdominal recurrences predominating [20, 32−38]. The histologic feature of papillary architecture alone does not appear to increase the recurrence rate [35, 36], although some authors have suggested that this presents some increased risk [39, 40]. In patients with papillary serous histology, adjuvant radiation therapy would need to address the whole abdomen and is discussed later. Clear cell carcinoma has also been noted to have a higher recurrence rate [33, 41, 42].
The Role of Radiation in Operable Clinical Stage I Endometrial Adenocarcinoma
There have been numerous single-institution reviews and a few prospective, randomly assigned trials addressing the role of adjuvant irradiation, most of these reports based on the old FIGO (International Federation of Gynecology and Obstetrics) staging system that included stages IC, IIA and IIB. The uterine neoplasm staging system has been updated by FIGO and American Joint Committee on Cancer (AJCC) in 2010 and according to the new staging system, stage I now includes stage IA—that is tumor invades less than half of the myometrium, and stage IB—that is tumor invades one half or more of the myometrium. These changes were made because the survival rates of different stages in the previous staging system were similar [43, 44]. When combined with surgery, radiation can be given either before or after surgery. Advocates of preoperative irradiation state that the benefits include irradiating the tumor with an intact blood supply with a possible reduction in subsequent distant metastases and a questionable decreased risk of radiation side effects. Postoperative irradiation has the advantage of prior staging to help determine the need for irradiation and the areas at risk.
Aalders and coworkers [43] published a trial of 540 clinical stage I patients randomly assigned to postoperative vaginal irradiation with or without additional EBRT. The patients who received additional EBRT had a pelvic/vaginal recurrence rate of 1.9 vs. 6.9 % in patients who were not given additional irradiation. No survival advantage was seen with EBRT. With additional evaluation, the authors concluded that patients with grade 3 disease who had more than half myometrial invasion benefited significantly from additional EBRT. The authors also recommended irradiation in cases of vascular invasion, given the poor prognosis shown in these lesions. Piver and associates [44] reported their results from a prospective, randomly assigned trial in clinical stage I patients comparing hysterectomy alone vs. preoperative uterine irradiation or postoperative vaginal irradiation. They noted more vaginal recurrences in patients who had received a hysterectomy alone (7.5 %) than in patients treated before surgery (4.5 %); none of the patients treated after surgery had a vaginal or pelvic recurrence.
In multiple, nonrandomly assigned reviews, authors have attempted to define the role of radiation in stage I disease. Piver and colleagues [45] reported their results from a prospective trial using postoperative vaginal irradiation in patients with grade 1/2 disease who had invasion of less than 50 % and no other evidence of disease. Patients with grade 3 disease or deep myometrial invasion received postoperative EBRT (group II). No patient in group I had a recurrence, and only one patient in group II had a pelvic recurrence. Grigsby and associates [46] reported the results of a study of 858 clinical stage I patients, most of whom received preoperative intracavitary irradiation. Patients with deep myometrial invasion received EBRT. Only 1 % of these patients had an isolated pelvic recurrence, and 3 % had pelvic and distant recurrences. Nori and coworkers, [47] using vaginal and selected EBRT either before or after surgery, noted a significant reduction in recurrences and improvements in survival compared with those of historical control subjects who had received surgery alone. Similarly, excellent pelvic and vaginal control rates have been noted in multiple series combining surgery and radiation [48−54]. A survey of American gynecologic oncologists was undertaken to analyze surgical staging and its effect on adjuvant treatment recommendations in stage I endometrial carcinoma. For patients without lymph node metastasis, the majority of gynecologic oncologists recommended radiation for patients with grade 3 lesions or deep invasion or both. The recommendations for grade 1 and grade 2 lesions and lesions that are not deeply invasive were more variable [54].
To define the role of radiation therapy in intermediate-risk endometrial adenocarcinoma, the GOG performed a prospective, randomly assigned trial (GOG-99). All patients received complete surgical staging and were found to have stage IB, IC (corresponding to IA, IB in the updated FIGO staging), or II (occult) disease. The patients were randomly assigned to no additional therapy or 50.4 Gy of whole-pelvic radiation therapy. A total of 390 eligible patients were randomly assigned. The estimated 2-year, progression-free interval was 88 % in the nontreated group vs. 96 % in the radiation therapy group (p = 0.004). There were 17 pelvic/vaginal recurrences in the nontreated group vs. three in the radiation therapy group (two patients who refused radiation therapy). The estimated 3-year survival was 89 % in the no-additional-therapy group vs. 96 % in the radiation therapy group (p = 0.09). The 5-year survival rates, 92 vs. 86 %, though not significant, favored the radiation group. An unplanned subset analysis was conducted in an attempt to define a group of patients with increased risk of recurrence. This group, based on prognostic factors including high grade, advanced age, deep myometrial invasion, or lymphovascular space involvement was defined as high-intermediate risk (HIR). The 2-year cumulative incidence of recurrence was 26 % in the observation group vs. 6 % in the radiation group [55]. Results of a randomly assigned study from the Netherlands (PORTEC trial) were reported by Creutzberg and associates [56]. In this trial, patients were randomly assigned to pelvic radiation therapy (46 Gy at 2 Gy/fraction) vs. no further therapy. Eligibility criteria included any adenocarcinoma including papillary-serous and clear cell, postoperative FIGO stage I, grade 1 with deep (greater than 50 %) myometrial invasion, grade 2 with any invasion, and grade 3 with superficial (less than 50 %) invasion. Peritoneal cytology was recommended but not required. In all 714 patients were entered and evaluable. The majority of patients were histologically adenocarcinoma. Approximately one-third of patients were FIGO stage IB, grade 2. There were six grade 3 complications and one grade 4 complication in the radiation therapy group vs. one grade 3 complication in the surgery-alone patients. Five-year locoregional recurrences were noted in 14 % of the untreated patients vs. 4 % in the radiation therapy patients (p < 0.001). Overall 5-year survival was 85 % in the control group vs. 81 % in the radiation therapy group (p = 0.37). Following subsequent central pathology review, there was a substantial shift from grade 1 to grade 2 lesions that would not have been eligible for inclusion in the study. Exclusion of these cases from analysis yielded essentially unchanged results, with 10-year recurrence rates of 5 % for the radiation therapy group and 17 % for the control group (p < 0.001), and 10-year overall survival rates of 65 and 70 %, respectively. Additionally, a subset analysis was conducted on patients with at least two of three risk factors (grade 3 lesions, outer 50 % myometrial invasion, and age ≥ 60 years) who were found to have increased risks of locoregional relapse. The 10-year rates of locoregional recurrence in this high-risk group were 4.6 % in the radiation therapy group and 23.1 % in the control group [57]. Fifteen years follow-up update was released where 426 patients were still alive at the analysis date with median follow-up of 13.3 years. The 15-year actuarial locoregional recurrence rates were 6 % for patients who received EBRT compared to 15.5 % for those who did not (P < 0.0001), however, the difference in 15-year overall survival was not statistically significant (52 vs. 60 %, p = 0.14). A trend towards increased second primary cancer was noted (22 % for EBRT vs. 16 % p = 0.1). Multivariate analysis confirmed the relevance and importance of risk stratification for treatment selection, with high-risk patients as the best candidates to receive postoperative adjuvant radiation therapy [58]. The two randomly assigned studies, GOG-99 and the Postoperative Radiation Therapy in Endometrial Carcinoma (PORTEC) trial from the Netherlands, both seem to support the ability of radiation therapy to improve locoregional control in early stage endometrial cancer. This benefit is seen despite the inclusion of relatively lower-risk patients with stage IB disease. The GOG trial also notes a strong trend to an improved survival. The significantly improved locoregional control demonstrated by adjuvant radiation therapy in the PORTEC-1 trial was achieved primarily by a reduction in vaginal recurrence as compared to the control arm [17]. Vaginal brachytherapy (VBT) alone has been shown in many single-institution nonrandomized trials to result in a low rate of recurrence in properly selected patients [59−65].
The ASTEC and EN.5 trials were randomized trials in which 905 patients were randomized to adjuvant pelvic EBRT (40–46 Gy in 20–25 fractions) or no adjuvant EBRT. Thus far, the data have only been presented in oral presentation format at the ASCO 2007 annual meeting. VBT could be used regardless of the external beam randomization and was delivered as 4 Gy in two fractions (HDR) or 15 Gy via LDR. Treatment centers were required to decide in advance whether they would offer brachytherapy to all patients or to no patients. Brachytherapy was given to 52 % of patients in each arm. Morbidity was 56 % in the EBRT arm compared to 24 % in the no-EBRT arm. At a median follow-up of 46 months, the 5-year hazard ratio (HR) for radiation therapy for overall survival was 1.01 (p = 0.98). The 5-year HR for radiation therapy for disease-specific survival was 1.17. The HR for an isolated pelvic or vaginal recurrence was 0.53 for the group receiving EBRT. There is a small but significant decrease in pelvic recurrence with pelvic EBRT [66].
The PORTEC-2 was designed to compare postoperative EBRT to postoperative VBT in 427 patients with high-intermediate risk endometrial cancer. For this trial, HIR was defined as (1) age ≥ 60 and stage IC grade 1–2, (2) age ≥ 60 and stage IB grade 3, or (3) any age and stage IIA grade 1–2, or grade 3 with < 50 % myometrial invasion. At a median follow-up of 36 months, 3-year actuarial rates of vaginal relapse were 0.9 % in the VBT arm and 1.9 % in the EBRT arm (p = 0.97). The 5-year rates of vaginal recurrence were 1.8 % (95 % confidence interval [CI] 0.6–5.9) for VBT and 1.6 % (0.5–4.9) for EBRT (HR 0.78, 95 % CI 0.17–3.49; p = 0.74). Five-year rates of locoregional relapse (vaginal or pelvic recurrence, or both) were 5.1 % (2.8–9.6) for VBT and 2.1 % (0.8–5.8) for EBRT (HR 2.08, 0.71–6.09; p = 0.17). 1.5 % (0.5–4.5) vs. 0.5 % (0.1–3.4) of patients presented with isolated pelvic recurrence (HR 3.10, 0.32–29.9; p = 0.30), and rates of distant metastases were similar (8.3 % (5.1–13.4) vs 5.7 % (3.3–9.9); HR 1.32, 0.63–2.74; p = 0.46). There was no difference in overall (84.8 % (95 % CI 79.3–90.3) vs 79.6 % (71.2–88.0); HR 1.17, 0.69–1.98; p = 0.57) or DFS (82.7 % (76.9–88.6) vs 78.1 % (69.7–86.5); HR 1.09, 0.66–1.78; p = 0.74). Rates of acute grade 1–2 gastrointestinal toxicity were significantly lower in the VBT group than in the EBRT group at completion of radiotherapy (12.6 % (27/215) vs 53.8 % (112/208)). The authors concluded that VBT should be the treatment of choice for patients with high-intermediate risk of recurrence [67, 68].
Stage II Disease
Treatment of stage II disease ranges from radiation therapy alone to radical hysterectomy to a combination of surgery and radiation. Treatment of patients with stage II disease with radiation alone has generally resulted in much lower control and survival rates than when radiation and surgery have been combined [69]. In addition, patients with cervical disease detected before surgery have been noted to have a worse prognosis than those patients with occult disease [69]. Patients presenting with clinical stage II disease have commonly been treated with preoperative irradiation followed by extrafascial hysterectomy. The 5-year survival rates in patients who have received a combination of preoperative EBRT, intracavitary irradiation, and hysterectomy range from 69 to 88 % [70−75]. The local control rates in these series are excellent. Grigsby and colleagues [73] noted an 8.9 % overall pelvic failure rate. Bruckman and associates [71] noted no isolated pelvic failures and an overall pelvic failure rate of only 5 %.
Radical hysterectomy alone has also been advocated as the treatment of choice by some authors. Boente and coworkers [76] noted a lower recurrence rate and complication rate in patients undergoing radical hysterectomy compared with patients treated with radiation therapy and extrafascial hysterectomy. Arguments against radical hysterectomy have included the observation that many patients with endometrial cancer are elderly or obese and thus have significant comorbidities. In addition, if the decision to add EBRT is made after surgery, a higher complication rate can be expected. Given the high false-positive rates of endocervical curettage, radical hysterectomy should probably be considered only in cases that include gross cervical involvement. Parthasarathy et al. reviewed data of 3664 endometrioid carcinoma patients in stages IC (corresponding to IB in current staging system) and II from the National Cancer Institute database who were diagnosed and treated between 1998 and 2001. One thousand one hundred and seventy-five patients among them received adjuvant radiotherapy; their 5-year survival rate was 89.9 % compared with 87.8 % in those who did not receive radiation and that was statistically significant (P = 0.04). Furthermore, there was improvement in disease-specific survival rate in stage II patients among those who received radiation therapy (86.5 % compared to 81.9 %; P = 0.02). The benefit of radiation was more notable in patients with grade 3 disease and in those 70 years or older [77]. A treatment approach that has gained favor in patients with stage II disease is initial extrafascial hysterectomy with lymph node sampling and cytology followed by irradiation. This approach has resulted in patient survival rates comparable to those seen in patients who received preoperative irradiation and has also resulted in excellent pelvic control rates [25, 78, 79].
Stage III Disease and Stage IVA Disease
Stage III or stage IVA disease can be separated into clinical and pathologic. Multiple series have noted an increased recurrence rate when irradiation alone is used [80−82]. Patients with pathologic stage III disease have a better prognosis compared to patients with clinical stage III disease [83, 84]. The role of radiation in stage III/IVA disease needs to be individualized for the extent of disease in each particular patient. In postoperative patients with positive pelvic lymph nodes, adnexal disease, serosal or parametrial spread, vaginal metastasis, or bladder/rectal invasion, pelvic irradiation with or without a vaginal-cuff boost should be considered. Using this algorithm, most series report 5-year survival rates of approximately 40–50 % in patients with pathologic stage III disease [81, 82]. Local control is accomplished in the majority of patients. In certain situations, there may be a role for extended-field and whole-abdominal irradiation (WAI).
Extended-Field Irradiation
The use of extended-field irradiation is limited to patients at high risk for extrapelvic recurrence. The clearest indication appears to be in patients who have evidence of para-aortic lymph node metastases as the only evidence of disease outside the pelvis. Extended-field irradiation refers to irradiating the pelvis, the common iliac, and the para-aortic lymph nodes. Potish and associates [85] reported their results in irradiating 40 women, all of whom had evidence of para-aortic lymph node metastasis. They reported a 47 % 5-year survival in surgically staged patients, with only one severe complication. These results compare to a 10 % 5-year survival in previous series that did not use extended-field irradiation [86]. Rose and colleagues [87] compared 17 patients who received extended-field irradiation to nine who did not. The survival in the extended-field irradiation group was 53 % compared to 12 % in the non-irradiated group, despite one treatment-related death in the former group.
Whole-Abdominal Irradiation
The role of WAI in endometrial carcinoma remains controversial. Whole-abdominal irradiation has been used in a variety of patients ranging from those who received adjunctive therapy for high-risk surgical stage I disease [88] to those with intraperitoneal metastatic disease [89]. Whole-abdominal irradiation is used when there is a risk of intra-abdominal spread that may be impacted by treatment. A number of authors have advocated the use of WAI in treating surgical stage III patients. Gibbons and coworkers [88] noted a 57.8 % 7-year DFS in patients with surgical stage III disease who were treated with WAI. Potish and associates [90] also noted an excellent 5-year relapse-free survival of 90 % in patients with adnexal metastases or positive peritoneal cytology compared to zero in patients with macroscopic spread of cancer beyond the adnexa. The Gibbons article noted that three of a total of 27 patients treated with WAI had significant long-term bowel toxicity [88]. The Potish article noted that only one of 27 patients had significant long-term bowel toxicity, although these investigators used a lower dose of WAI [89].
Loeffler and colleagues [91] reported the Joint Center experience with the use of WAI in 16 patients. They concluded that patients with extensive extrauterine involvement, and sarcomas, did not appear to benefit from WAI and that it may have reduced intra-abdominal recurrence in only a small subset of patients. Smith and associates, [92] in an update of the Stanford experience, noted a 3-year DFS rate of 79 % with an overall survival rate of 89 % in patients with stage III or IV endometrial adenocarcinoma.
Chemotherapy
A phase II study was conducted by the Radiation Therapy Oncology Group (RTOG 9708) combining adjuvant pelvic radiation therapy with concomitant chemotherapy followed by chemotherapy in grade 2 or 3 endometrial adenocarcinoma with either > 50 % myometrial invasion, cervical stromal invasion, or pelvic-confined extra-uterine disease. Forty-six patients were enrolled with a median follow-up time of 4.3 years. Chronic toxicity was grade 1 in 16 %, grade 2 in 41 %, grade 3 in 16 %, and grade 4 in 5 %. Overall survival and DFS were 85 and 81 %, respectively. The 4-year pelvic, regional, and distant recurrence rates were 2, 2, and 19 %, respectively. There were no recurrences in patients with stage IC, IIA, or IIB disease. While patients with extrauterine stage III disease demonstrated a pattern of distant recurrence, this trial illustrates the potential of combined therapy in the postoperative treatment for patients with disease confined to the uterus [93]. A randomized phase III study in early stage high-risk endometrial cancer patients compared adjuvant radiation therapy with or without chemotherapy (NSGO-EC-9501/EORTC 55991). Eligible patients had surgical stage I, II, IIA (with positive peritoneal cytology only), or IIIC (positive pelvic lymph nodes only) and qualified for adjuvant therapy based on risk of micrometastatic disease. Radiation therapy consisted of EBRT to 44 Gy with or without a VBT boost. The HR for progression-free survival was 0.58 in favor of combined therapy (p = 0.046), which translated into an estimated 7 % absolute difference in progression-free survival from 75 to 82 % [94]. The GOG 122 trial randomized patients between whole-abdominal radiation therapy and chemotherapy with cisplatin and doxorubicin. A total of 396 patients with stage III or IV endometrial cancer were randomized to receive WAI (30 Gy in 20 fractions, with a 15 Gy boost) or chemotherapy with cisplatin and doxorubicin every 3 weeks for seven cycles, followed by one cycle of cisplatin. With a median follow-up of 74 months, the HR for progession of disease was 0.74 favoring the chemotherapy arm. The stage-adjusted death HR was 0.68, also favoring the chemotherapy group [3]. Mundt and coworkers [95] reported recurrence rates in 43 patients with stage I–IV endometrial cancer who received adjuvant chemotherapy alone. A recurrence rate of 67.4 % was seen, with a 3-year actuarial pelvic recurrence rate of 48.1 %. Thirty-one per cent of recurrent patients recurred in the pelvis alone. Given these results, adjuvant chemotherapy protocols in endometrial cancer should probably continue to incorporate locoregional radiation therapy.
Uterine Papillary Serous Carcinoma
As discussed previously, patients with uterine papillary serous carcinoma have a higher recurrence rate compared to those with other uterine adenocarcinomas; there is also a preponderance of upper abdominal failures in these patients [20, 32, 38]. This has led a number of investigators to attempt more aggressive adjuvant radiation therapy, including WAI. Published reports of studies using WAI in patients with uterine papillary serous carcinoma suggest a reduction in recurrence rates in early stage disease. Mallipeddi and associates [95] reported the use of whole-abdominal radiation on ten patients with uterine papillary serous carcinoma, five of whom were alive at follow-up. This study noted long-term control in patients with superficial myometrial invasion, with or without positive cytology, who received optimal radiation. As in a previous report, [96] vaginal recurrences were lower with a vaginal-cuff boost. Gibbons and coworkers [88] noted a 60 % long-term recurrence-free survival in a group of patients who received WAI therapy. The 5-year actuarial survival in patients treated with whole-abdominal radiation therapy was 86 % [97]. This is in contrast to the low 3-year survival in GOG-94 [98, 99]. Chemotherapy is also frequently used as an adjuvant therapy for papillary serous cancer. Table 1.2 reviews various series using whole-abdominal radiation.
Table 1.2
Clinical results of whole-abdominal radiation
Reference | No. of patients | % Serous histology | Survival (%) | Recurrence rate (%) | Follow-up (median months) |
|---|---|---|---|---|---|
Mallipeddi et al. [100] | 10 | 100 | 60 | 50 | 64 |
Frank et al. [96] | 9 | 100 | 55 | 67 | 25 |
Greer and Hamberger [89] | 31 | 63a (5 year) | 19 | > 24 | |
Gibbons et al. [88] | 56 | 18 | 64 (7 year) | 36 | 45 |
Loeffler et al. [91] | 16 | 50 (1.5 year) | 62.5 | 17 | |
Small et al. [97] | 30 | 47 | 86 (5 year) | 23 | 27 |
Potish et al. [90] | 27 | 0 | 71 | 25 | NS |
Smith et al. [92] | 48 | NS | 77 (3 year) | 40 | 37 (mean) |
Techniques of Radiation Therapy
Radiation can be delivered by means of external sources (EBRT), implanted irradiation (brachytherapy), or radioactive fluid. This section discusses EBRT and brachytherapy. Radioactive fluid instillation is occasionally done intraperitoneally most commonly as adjuvant therapy in ovarian cancer and rarely in patients with positive peritoneal cytology. Some work has been done using P37 in patients with endometrial carcinoma with positive cytology [101]; this work is not discussed further, however, because data are somewhat limited.
EBRT is used to irradiate areas thought to be at risk for disease recurrence, including the whole pelvis, the whole pelvis plus the para-aortic nodal region, and the whole abdomen. EBRT is produced by cobalt machines, linear accelerators, or with charged particle cyclotrons (i.e., protons). As the energy of radiation increases, the beam penetration also increases, making it possible to limit the peripheral radiation needed for delivery of a desired dose at depth. Because the pelvis has a relatively thick separation, higher energy beams are preferred. There are limited data regarding charged particle therapy and this form of therapy is beyond the scope of this chapter.
Whole-abdominal irradiation is used to irradiate the entire abdominal contents. With modern radiation machines, this usually can be accomplished with a single setup, treating with an anterior and posterior field. The total whole-abdominal dosage is usually limited to 2000–3000 cGy in fractions of 100–150 cGy per treatment. Vital organs may need to be shielded to limit the radiation dose. The kidneys should be shielded to limit the dose to approximately 1800 cGy; liver shielding should also be considered if the dose exceeds 2500 cGy. Whole-abdominal irradiation in endometrial cancer is usually followed by a boost to the pelvis, preceded in many situations by a para-aortic nodal boost.
Treatment of the para-aortic nodes can be accomplished with either separate fields matched to the pelvic field or in continuity with pelvic radiation fields. We prefer to use a single field to avoid problems of matching. The para-aortic nodes can be treated with a two- or four-field technique, generally to a total dosage of 4500 cGy at 180 cGy per fraction. If a two-field technique is used, care must be taken to ensure that the dose to the spinal cord is limited to less than 4500 cGy. If a four-field technique is used, the location of the kidneys must be verified to avoid exceeding kidney tolerance.
Whole-pelvic irradiation can be accomplished by either a two- or four-field technique using 3D conformal radiation therapy, with intensity-modulated radiation therapy or Tomotherapy. To avoid excessive maximal dosages, the two-field technique should be used only with high-energy beams. The two-field technique uses opposed anterior and posterior fields. The upper border of the field is generally placed at the L4-5 or L5-S1 interspace. If there is no disease extension into the vagina, the lower border should encompass one-half to two-thirds of the vagina. The lateral borders should be placed approximately 1.5 cm lateral to the bony pelvic rim. A marker should always be placed to indicate the location of the vaginal cuff/cervix or the most distal aspect of tumor extension. The four-field technique allows lateral shielding of structures that cannot be shielded in the anteroposterior field. In the four-field technique, the upper- and lower-field borders are identical to those in the two-field technique. The anterior border of the lateral field is placed at or anterior to the anterior pubic symphysis. The posterior border is placed at the S2-3 interspace unless tumor extension necessitates larger fields. With 3D conformal radiation therapy, currently the standard of care for radiation therapy, the CTV, defined as the area that is at risk for harboring microscopic metastatic disease, is outlined on a CT scan. Normal tissues, such as bladder, rectum, large intestine, and small intestine, are also outlined in the same manner. Anteroposterior, posteroanterior, and lateral field borders are defined to include the CTV while sparing as much normal tissue as possible. A dose volume histogram, or DVH, can then be created to define the amount of normal tissue receiving a certain critical dose if felt to be clinically important.
Pelvic radiation therapy technique is extremely important in treatment outcomes, especially in reducing short-term and long-term toxicity [102]. Barium should be given at the time of simulation to document the position of the small bowel [103]. Attempts to reduce the small bowel in the radiation field include placing the patient in the prone position with a full bladder with or without abdominal compression. Patients should always be treated with a full bladder to move as much of the small bowel as possible out of the pelvic field. The total pelvic radiation therapy dosage typically is 45–50 Gy for adjuvant therapy.
Intensity-modulated radiation therapy (IMRT) is a radiation technique, which is currently increasingly used for treatment of gynecologic malignancies including endometrial cancer especially in adjuvant settings to minimize gastrointestinal complications. This technique allows for decreased radiation doses to critical structures such as bone marrow or small bowel while continuing to treat the tumor to the same dose. Several small trials have showed an improved toxicity profile with IMRT [104−106]. A recently closed trial, RTOG 0418, was designed to assess the utility, efficacy, side effects, and control and survival rates when IMRT is used for postoperative endometrial and cervical cancer.
Brachytherapy refers to the placement of a radioactive source in or near the desired treatment volume. This allows a higher local radiation dose and spares surrounding normal tissues. The two main forms of deliver of brachytherapy are the LDR and the HDR techniques. The LDR technique uses isotopes that deliver radiation with a dose rate of approximately 40–100 cGy/h to the prescribed target. HDR brachytherapy, which delivers approximately 200 cGy/min, can be performed on an outpatient basis. There is a significant biologic difference between LDR and HDR brachytherapy: HDR delivery has a higher “effective” radiation dose for the same nominal LDR dose. Therefore, the delivered HDR doses must be adjusted lower to give the same effective LDR treatment. Pulsed Dose Rate (PDR) brachytherapy attempts to eliminate the unfavorable radiobiology of the High Dose Rate Brachytherapy while maintaining the ability to optimize finely the dose distribution and eliminate the personnel exposure to radiation. Biologically, since each fraction comes before the complete repair of the sublethal cellular damage, the tissue experiences the radiation as almost continuous, mimicking LDR brachytherapy. Although, this approach incorporates the biological advantage of Low Dose Rate brachytherapy and the optimization advantage of the High Dose Rate brachytherapy, it also has many disadvantages including inpatient treatments, lack of applicator stabilization, and possibility of mechanical failure. PDR brachytherapy presents opportunities to potentially improve brachytherapy, but it also come with detriments. Although PDR has prospered in Europe and Asia, unfortunately in the USA it has floundered because the Nuclear Regulatory Commission (NRC) requires that a physicist and/or radiation oncologist be present throughout the treatment, which is almost impossible to accomplish in a long treatment schedule in a hospital setting [107]. The isotopes used in LDR treatment typically include cesium-137 or radium-226. Radium-226 has fallen out of favor because of radiation safety issues. Cesium-137 has a half-life of 30 years, allowing reuse of a source over a long period, although periodic calibration to allow for decay is necessary. HDR and PDR treatments typically use an iridium-192 source that needs frequent recalibration and replacement. Iridium-192 can also be used as an LDR isotope. Typically, in most gynecologic applications of brachytherapy, the sources of radiation are left in place temporarily and then removed. This is the case in most LDR applications and all HDR applications. Permanent LDR brachytherapy procedures have a limited use in gynecologic malignancies and are not discussed further. The sources of radiation are, in almost every case, afterloaded into a hollow radiation carrier. This permits some planning before determining the strength of radioactive isotope to use and significantly reduces radiation exposure during placement. The carriers used for afterloading can be divided roughly into those used to treat the intact uterus and those used after surgery. The uterus may be treated with a tandem alone, as is done in treating cervical cancer. Treating the uterus with a tandem alone may underdose the thicker sections of the myometrium. The use of duel-curved tandems or three tandem applicators, as noted above, has been shown to have good outcome data for toxicity, recurrence, and survival for unresectable disease [18]. Heyman [108] originally described using multiple radium capsules packed into the uterus to stretch and thin the wall to improve the dose distribution. Simon and Silverstone [109] later developed afterloading capsules to decrease radiation exposure during placement.
Brachytherapy dose is defined either in terms of actual dosage delivered or in terms of total milligram-hours, which is simply derived by multiplying the total milligrams of equivalent radium by the total number of hours of the implant. The doses of radiation used when delivered before surgery with planned hysterectomy typically range from 2500 to 4000 mg-h to the uterus using a tandem or Simon–Heyman capsules and colpostats to deliver 1900–2000 mg-h (6000–6500 cGy vaginal surface dose) to the upper vagina. In some patients, 50 Gy of postoperative EBRT is added, with the whole-pelvic dosage limited to approximately 2000 cGy by the addition of a midline shield. When definitive radiation is delivered without planned hysterectomy, uterine milligram-hours range from 3000 to 10,000, depending on whether EBRT is also delivered [4−9]. Although not commonly reported, the point A dose (i.e., the dose defined as 2 cm superior and 2 cm lateral to the external os) is approximately 7500–8500 cGy [4, 6]. HDR is generally delivered in a fractionated manner, with an attempt to deliver biologically equivalent dose to the traditional LDR implants.
Posthysterectomy VBT is generally delivered with a vaginal cylinder or with ovoids. The dose delivered with low-dose brachytherapy alone tends to be prescribed at the vaginal surface. Doses range from 6000 to 7000 cGy [43, 48, 49]. The use of postoperative high-dose brachytherapy is becoming more common, allowing outpatient treatment. A common dose schedule is 2100 cGy divided into three fractions of 700 cGy and prescribed to 0.5 cm from the vaginal mucosal surface [47]. There is quite a bit of variation in the dose schedule for high dose rate radiation amongst radiation oncologists [110]. The ideal timing of postoperative radiation therapy is not known. There is support for initiating postoperative irradiation within 6 weeks after surgery. A higher local failure rate was seen with a delay of longer than 6 weeks [111]. Given the time needed to initiate treatment planning, patients for whom postoperative irradiation is being considered should be referred immediately to the radiation oncologist to prevent nonmedical delays in the initiation of therapy. The need for a vaginal-cuff boost after postoperative EBRT recently has been questioned by a number of investigators [111, 112]. Numerous large studies have consistently used vaginal-cuff boosts with excellent long-term results [46, 47]. In addition, at least one nonrandomly assigned review noted improved local control with the addition of a vaginal-cuff boost to postoperative EBRT [113]. The number of absolute vaginal-cuff recurrences prevented by a vaginal-cuff boost is probably small. Most institutions utilize HDR vaginal-cuff boosts and have used a 600 cGy vaginal surface dose for two to three fractions. Other institutions deliver a higher total vaginal mucosal dose and limit the mid-pelvis external dose by using a midline shield [46].
Recurrent Disease
Locoregionally recurrent endometrial cancer can be cured with radiation therapy, even when resection is a reasonable option but may be less desirable because of potential surgical complications owing to common comorbidities among uterine carcinoma patients such as obesity, hypertension, and others. Results tend to be best in patients with vaginal-cuff recurrences and without previous irradiation. Curran and colleagues [114] reported on 55 patients with isolated vaginal recurrences who were treated with definitive irradiation. Patients with vaginal mucosal recurrence had a 3-year actuarial survival and a local control rate of 85 and 100 %, respectively. This compared with a 13 % 3-year actuarial survival and a 0 % local control rate in patients with sidewall involvement at the time of recurrence. The 5-year survival rate overall was 48 % in patients who did not receive previous irradiation compared to 16 % in patients who were receiving their second course of radiation therapy. Other authors have seen similar results [115−120]. The PORTEC trial also noted a 3-year survival of 69 % after vaginal recurrence compared to 13 % after pelvic or distant relapse [56]. Other prognostic factors noted included histologic type of recurrence, [117] time to recurrence, [116], and tumor size [120]. Site of recurrence is also to be considered: data from the PORTEC trial demonstrated that the 3-year survival rate among women with pelvic recurrence was only 8 % compared to 73 % for women with isolated vaginal recurrence. In this update, treatment of vaginal relapse was effective with 89 % complete response and 5-year survival rate of 65 %. The survival rate was similar for patients with pelvic recurrence to those with distant metastases [121]. The exact technique used in salvage irradiation needs to be individualized for each patient. Generally, a combination of EBRT and brachytherapy should be used. Because the normal anatomic barrier of the uterus does not confine recurrent disease, EBRT to sterilize nonpalpable disease should probably always be part of the planned therapy.
Uterine Sarcomas
Uterine sarcomas tend to behave in a more malignant fashion than do endometrial cancers. The three most common histologic variants of uterine sarcomas are endometrial stromal sarcoma (ESS), leiomyosarcoma (LMS), and carcinosarcoma. As in endometrial adenocarcinomas, surgery is the preferred primary therapy in uterine sarcomas. A number of institutions have reviewed their experience in patients who have received adjuvant radiation and compared the results to patients who underwent surgery alone. The data are presented in some series as uterine sarcomas, and in others the histologies are divided among carcinosarcoma, LMS, and ESS. Given the selective use of radiation in these trials, a bias towards irradiating patients with poor prognostic features would be expected. Despite this bias, there is significant evidence to support the use of adjuvant radiation in many patients. There seems to be a general consensus that postoperative radiation therapy improves local control in carcinosarcomas. Some reviews support an improvement in survival, while others do not [122−129].
A randomized trial was recently reported in which 224 patients who underwent total abdominal hysterectomy bilateral salpingo oophorectomy (TAH-BSO) and were randomized to adjuvant EBRT (50.4 Gy in 28 fractions) or no further therapy. Results showed improved locoregional control with adjuvant EBRT (p = 0.004), but did not show an improved overall survival or progression-free survival [130].
LMSs tend to have a higher propensity for distant metastasis, and it would, therefore, follow that local adjuvant treatments may have less of an influence on survival. There is evidence in some series for an improvement in local control with the addition of adjuvant radiation [131, 132]. Hornback and coworkers [133], conversely, reviewing the use of radiation in GOG-20, did not find a difference in first recurrence rates with the use of adjuvant radiation in LMS, although an improvement in pelvic control was seen in the mixed mesodermal sarcomas. There is less support regarding improvement in survival. At least one institution noted no improvement in survival with adjuvant radiation when treating LMSs with low mitotic activity [134].
A GOG phase III study [135] examined the postoperative efficacy of postoperative WAI with 1 Gy BID or 1.5 Gy daily to a total dose of 30 Gy compared to adjuvant cisplatin, ifofsamide, and mesna (CIM). The study focused on patients with carcinosarcoma and included all stages of disease. In all, 232 patients were randomized with 43 % of the patients being stage I–II, and 45 % stage III. There were a total of 112 recurrences, with 60 occurring in the WAI group and 52 in the CIM group. There were no significant differences in the number or site of recurrences. However, there were slightly more vaginal recurrences with CIM and slightly more abdominal recurrences with WAI. There was no significant survival difference.
ESSs have traditionally been divided into low grade and high grade. The National Comprehensive Cancer Network (NCCN) guidelines have modified the classification of ESSs, so that the group previously referred to as high-grade ESSs is now known as high-grade undifferentiated sarcomas, and the group previously referred to as low-grade ESSs is now known simply as ESS. Patients with low-grade ESS tend to have a favorable prognosis, and there is little evidence that in early stage disease, adjuvant radiation would offer a benefit [136]. There is evidence that adjuvant radiation may improve local control in patients with high-grade ESS [122, 128] and possibly survival [128, 137].
In a series of patients with ESS reported by Weitmann and associates [138], a 93.8 % 5-year local control rate was seen in patients who received surgery and radiation therapy, with the majority of patients having high-grade disease. The actuarial overall survival at 10 years was 52.8 %.
A number of publications have looked at uterine sarcomas without dividing the patients into separate histologic categories. A report by the Grup Oncologic Catala-Occita reviewed their experience in 103 patients with uterine sarcomas. A local control and survival advantage was seen with adjuvant radiation [139]. The Curie Institute also reported on uterine sarcomas and found an improvement in local control with the addition of radiation in high-grade tumors [140].
Given the overall rarity of uterine sarcoma, the above discussion basically focuses on earlier stage disease. The use of adjuvant radiation in advanced disease is based on even more limited data and extrapolations from endometrial adenocarcinoma results. There is a need for open dialogue between patient and physician, and also for further studies investigating the role of multimodality therapy.
Conclusion
Radiation plays a prominent role in the treatment of uterine tumors. Its most common role is in the adjuvant setting after hysterectomy. There may also be a role for adjuvant irradiation in some uterine sarcomas. When applied properly, radiation can contribute to tumor control with acceptable rates of serious complications.
Cervical Cancer
Cervical cancer is a major cause of morbidity and mortality worldwide, with an estimated incidence of over 11,000 new cases in the USA alone and much higher incidence in developing nations [141]. It is the third most common gynecologic malignancy and cause of death among gynecologic cancers in the USA [1].
Bulky Stage IB and IIA Disease
Women with bulky stages IB2 and IIA cervical cancer have a higher local failure rate and worse survival than those with smaller volume disease. Surgery as a sole treatment modality results in as high as 30 % relapse rate [142, 143]. Unfortunately, we still lack strong predictive molecular biomarkers that would most likely identify those at higher risk for relapse and provide this patient category with some benefit from individualized targeted therapies. Grag et al. suggested that pretreatment nuclear expression of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) might be associated with a poor outcome for cervical cancer patients treated with chemoradiation [144]. The optimal management strategies of women with primary tumors measuring ≥ 4 cm in diameter include: (1) primary chemoradiotherapy, (2) neoadjuvant chemotherapy, followed by radical hysterectomy with or without subsequent chemoradiotherapy, or (3) primary radical hysterectomy and lymphadenectomy followed by tailored RT with concomitant chemotherapy.
Concurrent Chemoradiation
Historically, Primary radiotherapy (RT) has usually been the treatment of choice for women with bulky stage IB2 and IIA2 cervical cancer. In a review of 1352 patients with stage IB disease treated with RT alone at the M. D. Anderson Cancer Center and followed for a median of 12.2 years, rates of central and pelvic tumor control and disease-specific survival for tumors < 5 cm were 99, 97, and 88 %, respectively, and for tumors between 5 and 7.9 cm were 93, 84, and 69 %, respectively [145]. However, among the 66 patients with tumor size > 8 cm, outcomes were less favorable (central and pelvic control and disease-specific survival rates were 69, 57, and 40 %, respectively). Furthermore, for patients with tumors in the 5–7.9 cm category, outcomes were significantly better for those with exophytic as compared to endocervical morphology (disease-specific survival 76 vs. 66 %, respectively). If primary RT is utilized, concomitant cisplatin during RT provides additional benefit over RT alone [146, 147]. Much of the data supporting chemoradiotherapy over radiotherapy alone come from trials conducted in the setting of locally advanced disease. Thus, when definitive RT is chosen, cisplatin-based chemoradiotherapy rather than RT alone is indicated. Timely completion of RT is essential for good outcomes, whether chemotherapy is used or not [148−152]. The importance of time to complete RT to overall outcomes was illustrated in a series of 1224 women with cervical cancer treated with definitive RT for stage IB–III disease [148]. Treatment courses extending beyond 9 weeks resulted in significantly higher rates of pelvic failure and poorer disease-specific survival at 10 years as compared to those whose treatment was administered over a shorter time period. Similar findings were noted in a pattern of care study involving 837 women undergoing RT for stages I–III cervical cancer [149]. As compared to a total treatment time of 6 weeks or less, those treated over 10 weeks or more had significantly higher rates of 4-year in-field recurrence (20 vs. 4 %, respectively). These data are retrospective rather than from prospective trials, and it is possible that longer treatment duration may be a surrogate for the presence of unfavorable tumor or patient characteristics. Nevertheless, in general, RT should be completed within 56 days, if at all possible. From a radiobiologic standpoint, it is likely that similar considerations apply to patients undergoing chemoradiotherapy, as well.
Many of these bulky tumors extend laterally beyond the tumoricidal isodose curve of the brachytherapy application; further, they may contain hypoxic central areas, which do not respond well to RT. These observations provide the rationale for some to recommend an extrafascial hysterectomy following chemoradiotherapy, since approximately one-half of these specimens harbor residual disease, even if concomitant chemotherapy is administered [153, 154]. While many studies find that pelvic recurrence rates are lower than expected (2–5 vs. 15–20 %) in women who have postradiotherapy hysterectomy, the impact of surgery on extrapelvic recurrence and survival is less well established [155−159]. A randomized study comparing RT with and without extrafascial hysterectomy in 256 women with bulky IB2 disease showed a lower local recurrence rate in the surgery arm (14 vs. 27 %), but the difference was not statistically significant [159]. Unfortunately, the study was hampered by the delivery of suboptimal doses in the RT alone arm (87 % received 78–80 Gy) with 51 % receiving RT over a protracted treatment period (> 60 days). Furthermore, concurrent chemotherapy was not administered. Despite the difference in local disease control, survival was similar in both groups. It has been suggested that the presence of residual local disease on cervical biopsies performed under anesthesia 8–10 weeks after the completion of chemoradiotherapy may serve to identify those women who may benefit from hysterectomy [160].
Neoadjuvant Chemotherapy Followed by Surgery
This is an acceptable treatment approach in communities with limited access to radiation therapy facilities in women with tumors, which would not otherwise be considered surgically resectable. Cisplatin-based chemotherapy is the mainstay in treatment; cervical squamous cell carcinoma is known to be a cisplatin-chemosensitive cancer. A prospective trial directly compared surgery (followed by adjuvant RT) with or without neoadjuvant chemotherapy (three courses of cisplatin 50 mg/m2, vincristine 1 mg/m2, and bleomycin 25 mg/m2 on days 1–3, at 10-day intervals) in 205 women with stage IB disease > 2 cm in diameter. Sixty-one patients in the study group and 56 in the control group had bulky stage IB tumors. Neoadjuvant chemotherapy was associated with a 90 % objective response rate, a higher likelihood of resectability with negative margins (100 vs. 85 %), and a significant decrease in the rate of pelvic failure, but only a trend towards better survival (82 vs. 77 %) [161]. Other studies either did not show any significant advantage for the neoadjuvant approach [162−164], or utilized suboptimal treatment in the control arm (RT instead of concurrent chemoradiation) [165].
However, a Cochrane meta-analysis of six trials enrolling 1036 women demonstrated statistically significant progression-free survival (HR 0.76, 95 % CI 0.62–0.94) in favor of neoadjuvant chemotherapy. However, this did not result in significant overall survival benefit, or decrease in local or distant recurrence. Furthermore, the analysis included significantly dissimilar clinical trials [166]. A phase III trial in patients receiving paclitaxel plus cisplatin and ifosfamide as compared to cisplatin and ifosfamide alone (48 vs. 23 %), showed significantly higher, optimal response rates, defined as surgical specimen residual disease with < 3 mm of stromal invasion, in the three-drug arm, but yet overall survival was not significantly different [167].
Primary Surgery
The advantage of this approach includes accurate pathological determination of the extent of the disease and subsequent individualized tailored adjuvant therapy, and the potential for resection of bulky metastatic lymph nodes, which may improve prognosis [168, 169]. In a large study comparing patients with stage IB1 and IB2 cervical cancers managed by primary radical hysterectomy, the prognosis of stage IB cervical cancer was best determined by lymphovascular space invasion and depth of invasion, not tumor size as staging criteria would suggest [170]. These factors are best determined pathologically after radical hysterectomy. For the subset of patients for whom radical hysterectomy is the sole treatment required, treatment time is shortened and acute and late radiation sequelas are avoided. Primary radical hysterectomy will also avoid the difficulties of determining if there is viable tumor left after completion of primary chemoradiation. Also, the potential for preservation of ovarian function in young women (although this is frequently not successful) and for prevention of radiation-associated vaginal stenosis, may be an important advantage of primary surgical management. A primary surgical approach is mandatory in the setting of an undiagnosed coexistent pelvic mass, or anatomic alterations that make optimal RT difficult. It might also be beneficial in patients with acute or chronic pelvic inflammatory disease which is a relative contraindication to concurrent chemoradiation [171]. Furthermore, if patients are poorly compliant with Radiation Therapy or if expert Radiation Therapy or a Brachytherapy facility is not available, primary radical hysterectomy should be performed.
Primary Surgery vs. Chemoradiation
This is an unpopular approach for treatment of bulky cervical cancer because most of the patients will still require postoperative RT or concurrent chemoradiation [172]. It might also result in higher morbidity and mortality in this patient subset [173−175]. In an RTOG trial of 367 women with stage IB or IIA cervical cancer randomly assigned to pelvic or pelvic plus para-aortic RT, the estimated cumulative incidence of grade 4 and 5 complications was 11 % in women who had postoperative RT vs. only 2 % in those who received RT alone [176]. However, with the current advances in surgical technique, there has been a significant decline in the rates of postoperative morbidity. GOG 92 trial randomly assigned 277 patients with intermediate-risk factors to pelvic RT vs. no further therapy following radical abdominal hysterectomy [177]. The rates of grade 3 or 4 complications involving the gastrointestinal and urogenital tracts in the RT group were 2.3 and 3.1 % vs. 0 and 1.4 %, respectively. Similarly, in GOG 109, a randomized trial of adjuvant RT vs. chemoradiation following radical hysterectomy in 243 patients with high risk factors, rates of posttreatment small bowel obstruction in the chemoradiation and RT alone groups were 3 and 2 %, respectively [178]. However, neither of these trials specifically enrolled patients with stage IB2 cancers.
Locally Advanced (Stage IIB, III, IVA) Disease
Definitive concurrent cisplatin-based chemoradiation therapy is the standard of care in locally advanced cervical cancer; however, a majority of those with recurrences have a poor prognosis despite improving salvage therapies [179−183]. Nodal involvement, particularly of para-aortic nodes, is the most important adverse prognostic factor, reducing survival by one-half. However, while the presence of lymph node metastases does not change the FIGO staging of cervical cancer, it significantly impacts the prognosis of these patients [179, 184]. Decades ago, it was thought that the lymphatic spread of cervical cancer advances in an orderly fashion, starting at the obturator lymph nodes and then progressing to the common iliac nodes, and the para-aortic nodes [185]. However, the implementation and utilization of the sentinel lymph node mapping technique clearly showed that not only any pelvic lymph node but also para-aortic lymph nodes might be the first site of metastases [186, 187]. Bader et al. [188] reported the variable pattern of first site of lymph node metastases in 619 invasive cervical cancer patients. Of 61 patients with one positive lymph node (10 %), the external iliac (43 %) and obturator (26 %) regions and the parametrium (21 %) were the most commonly involved pelvic lymph node sites with solitary metastases, and isolated metastases were reported to common iliac (7 %), presacral (1 %), and para-aortic nodes (1 %). Of 59 patients with two positive lymph nodes (10 %) at any location, patients had one parametrial and one pelvic node involved (32 %), two ipsilateral positive nodes (31 %), one positive lymph node on both sides of the pelvis (27 %), and two positive nodes within the parametrium (10 %) [188]. Several studies have shown that advanced FIGO stage, and increased depth of invasion increase the risk of lymph node metastases [189−191].
Controversies exist regarding nodal staging. Some institutions implement pretreatment staging lymphadenectomy as a standard institutional protocol while others rely mainly on imaging studies. Institutions where staging lymphadenectomy is a routine practice believe that CT and magnetic resonance imaging (MRI) are poor approaches in detecting small volume metastatic disease (< 1 cm), and doubt the specificity of [18] F-fluorodeoxyglucose positron emission tomography (PET) scanning [192]. One hundred eighty-four patients with stages IB2–IVA cervical cancer reported by Leblanc et al. underwent pretreatment laparoscopic staging procedures, including transperitoneal abdomino-pelvic exploration and extraperitoneal bilateral infrarenal para-aortic lymph node dissection. Twenty-four per cent of women with clinical stages IB2 and IIA cervical cancer were found to have positive para-aortic lymph node metastases that resulted in extending their radiation field, while sparing 75 % with stages IIB–IVA disease overtreatment. The authors confirmed the superiority of laparoscopic staging lymphadenectomy compared to CT or MRI in identifying patients with para-aortic lymph node metastases [192]. Utilization of laparoscopic lymphadenectomy resulted in improved staging lymphadenectomy-induced adverse events vs. the open extraperitoneal approach [193−196]. Other studies reported possible survival advantage of lymphadenectomy especially in patients with bulky nodal disease [197, 198].
As the only gynecologic malignancy that is still staged clinically, it should be noted that the accuracy of cervical cancer staging is only 60 %, with most errors related to undiagnosed lymph node metastases [199]. The sensitivity, specificity, positive predictive value, and negative predictive value of CT were only 34, 96, 60 and 91 % [200, 201]. Other reports showed the limitations of CT and MRI in diagnosing any microscopic lymph node metastases and reported 20–50 % failure in detecting macroscopic lymph node metastases. However, while PET scanning provided a better detection rate, its sensitivity did not exceed 86 % [202−208]. A GOG study evaluated the treatment outcomes of cervical cancer patients who had negative para-aortic lymph nodes identified by surgical staging vs. radiographic clinical staging prior to definitive chemoradiation. The analysis included patients who participated in 1 of 3 phase III GOG trials (GOG 85, GOG 120, and GOG 165). All patients had FIGO stage IIB–IVA disease without evidence of para-aortic lymph node metastases and received definitive cisplatin-based chemoradiation. The study included 555 patients who underwent surgical staging and 130 patients who underwent radiographic evaluation. Stage III and IV patients who underwent surgical staging had better 4-year progression-free survival (48.9 vs 36.3 %) and overall survival (54.3 vs 40 %). In multivariate analysis, the radiologic only staging was associated independently with a poorer prognosis compared with the surgical staging (for disease progression: HR, 1.35, 95 % [95 % CI], 1.01–1.81; for death: HR, 1.46, 95 % CI, 1.08–1.99). The study concluded that surgical staging might provide better prognosis [199].
Elective para-aortic radiation therapy in locally advanced cervical cancer patients who did not undergo surgical staging has been investigated in a number of controlled randomized clinical trials. RTOG randomly assigned 337 stage IIB cervical cancer patients to pelvic radiation therapy with or without 45 Gy to the para-aortic region and the 10-year cumulative incidence of death due to cervical cancer was estimated as significantly higher in the pelvic-only arm (P = 0.01). There was statistical significant 10-year overall survival difference in favor of the extended-field radiation therapy arm (55 vs 44 %, P = 0.02) but no difference regarding the DFS (40 vs 42 %). A higher percentage of local failures were salvaged on the extended-field arm compared with the pelvic-only arm (25 vs 8 %). This study also reported a trend, that was not statistically significant, for higher cumulative incidence of grade 4 and 5 toxicities among patients receiving extended field (8 %, 6 %) vs. pelvis only (4 %, 1 %) radiation therapy (p = 0.06, 0.24), respectively. Patients with a history of abdominal surgery prior to the extended-field radiation therapy had higher incidence of grade 4 and 5 complications (11 vs 2 %) [176]. Of 441 cervical cancer patients with either stage I and IIB disease with proximal vaginal and/or parametrial involvement and positive pelvic LNs either on lymphangiogram or at surgery, or stage III regardless of pelvic node status on lymphangiogram randomized in an European Organisation for Research and Treatment of Cancer (EORTC) study to whole-pelvis radiation therapy with or without extended field to include the para-aortic lymph nodes (45 Gy). The study did not show any statistical significant advantage for the extended-field approach; however, the study was criticized for the relatively inferior 4-year DFS (51 %). The authors noted statistically significant higher incidence of para-aortic metastases and distant metastases without tumor at pelvic sites among patients who did not receive para-aortic region radiation, but this did not result in any DFS or overall survival advantage [209]. RTOG 90-01 randomized 403 patients with locally advanced cervical cancer and clinically negative para-aortic LNs to extended-field radiation therapy (EFRT) vs. concurrent chemoradiation. All patients received LDR brachytherapy boost. At a median follow-up of 43 months, the 5 year OS was 73 vs. 58 % (P = 0.0004), and DFS was 67 vs. 40 % (P < 0.001) in favor of the concurrent chemoradiation. Furthermore, significantly less distant metastases (P < 0.001) and locoregional recurrences (P < 0.001) events were reported among patients receiving concurrent chemoradiation. There was no significant difference regarding the treatment-induced adverse events in both arms [181]. Updated results at a median follow-up of 6.6 years for the 228 surviving patients showed statistical significance DFS advantage among patients receiving concurrent chemoradiation (all stages) and OS stages IB–IIB disease with a trend for higher OS in stages III and IVA disease [180]. Currently, standard whole-pelvis chemoradiation is a standard of care among such patients with clinically negative para-aortic lymph nodes; however, a randomized controlled trial comparing extended-field chemoradiation vs. whole-pelvis chemoradiation in such patients would be worthwhile.
Many studies have investigated the efficacy and toxicity profile of EFRT in patients with proven para-aortic nodal metastases. Early studies demonstrated unacceptable rates of treatment-induced adverse events when utilizing EFRT concurrently with chemotherapy. Of 29 cervical cancer patients with biopsy-proven para-aortic nodal metastases who received hyperfractinated EFRT (1.2 Gy per fraction, twice daily) concurrent with 2–3 cycles every 3 weeks cisplatin-based chemotherapy and boosted by brachytherapy, 25 patients (86 %) completed the treatment course. Acute grades 3 and 4 chemotherapy-induced adverse events were 48 and 28 %, and radiation induced were 21 and 28 %, respectively. One patient died of grade 5 adverse events during the treatment course [210]. Updated results showed that the grade 3 and 4 late toxicity were 7 and 17 %. The 2- and 4-year OS were 46 and 29 %, and the probability of disease failure at any site was 46, 60, and 63 % at 1, 2, and 3 year, respectively. The authors concluded that the hyperfractionated EFRT with concurrent chemotherapy resulted in unacceptable toxicity but no advantage regarding patient’s survival or tumor control when compared to the standard fractionation [211].
A phase 2 RTOG trial that included cervical cancer patients with para-aortic or high common iliac nodes randomized to receive EFRT with concurrent weekly cisplatin and brachytherapy boost vs. same treatment plus amifostine aimed at reducing radiation-induced toxicity. Arm 1 included 26 patients who did not receive amifostine; the acute and late grade 3/4 toxicity rates were 81 and 40 %, respectively, and the estimated DFS and OS at 18 months are 46 and 60 %, respectively [212]. Arm 2 with amifostine included 15 patients after exclusions for ineligibility or withdrawing consents; the acute and late grade 3/4 toxicities were 87 and 20 %, respectively, and the estimated median survival was 34.8 months [213]. Similarly, a GOG study included 86 cervical cancer patients with para-aortic nodal metastases assigned to receive EFRT with concomitant cisplatin-based chemotherapy and brachytherapy boost. Acute grade 3–4 toxicities were gastrointestinal (18.6 %) and hematologic (15.1 %). The 3-year OS and PFI rate were 39 and 34 %, respectively [214].
Recently, IMRT has been increasingly utilized, aiming to improve the toxicity profile of EFRT in high-risk patients, limit the radiation dose to OARs (gastrointestinal (GI) and genitourinary (GU) tracts), and safely permit escalation of the radiation doses to involved pelvic and para-aortic lymph nodes beyond 55 Gy [105, 215−218]. Of 22 cervical cancer patients who received extended-field IMRT concurrently with chemotherapy, none of the patients experienced acute or subacute grade 3 or 4 GI or GU toxicity [217]. Another study included thirty-six patients with Stage IB2-IVA cervical cancer treated with extended-field IMRT with concurrent chemotherapy showed acute grade ≥ 3 GI, GU, and myelotoxicity of 1, 1, and 10 patients, respectively and late (2 year) grade ≥ 3 toxicity in 10 % of the patients. The 2-year actuarial locoregional control, DFS, and OS were 80, 51, and 65 % respectively [218]. Marnitz et al. compared IMRT delivered by helical tomotherapy (HT) compared to conventional IMRT concurrent with chemotherapy, and found that HT significantly improved the target comformity, homogeneity and OAR sparing. However, more evidence is required before adopting this approach [219]. A dosimetric comparison of IMRT, passive scattering proton therapy (PSPT), and intensity-modulated proton therapy (IMPT) to the para-aortic (PA) nodal region in advanced gynecological malignancies . All plans created included IMRT to pelvis nodes with either PSPT or IMPT to para-aortic nodes with optimization aimed to deliver 50.4 Gy. Both PSPT and IMPT resulted in statistically significant decrease in doses to OARs; namely, small and large intestines and kidneys while maintain appropriate coverage to the planning target volume. However, this looks like a promising data, clinical studies are required to provide enough evidence to adopt such therapy [220].
Radiation Therapy Techniques
External Beam Radiation Therapy
Historically, EBRT to the pelvis was based on the bony landmarks that included the entire pelvis. This approach minimized any geographical miss but meanwhile it resulted in high incidence of treatment-induced adverse events due to the involvement of the organs at risk within the radiation field. Two-dimensional, fluoroscopic radiographs assisted the planning. The whole pelvis was usually treated with 6–15 MV of X-ray via anterior and posterior parallel fields or box fields. After an EBRT dose of 44–50 Gy in 22–28 fractions over 4.5–5.5 weeks, and patients with locally advanced disease were boosted to 54–60 Gy, with central shielding [221].
With the advancement in RT and the development of CT based treatment planning, 3D conformal radiation therapy (3DCRT) has replaced the two-dimensional approach and became a standard of care almost everywhere. This approach decreased the treatment-induced adverse events and provided better coverage of the target volumes. MRI, or PET images can be fused with the CT simulation images for better localization of the tumor and more conformal coverage to avoid any possible marginal miss. The implementation of the IMRT technology further reduced the organs at risk of treatment toxicity [222−227]. Furthermore, this did not result in higher rates of in-field failures [222, 223, 227]. Guidelines on CTV definitions for a number of tumor sites including the postoperative gynecological setting have been published [228, 229]. Still, the volumes for definitive radiation therapy for cervical cancer patients varies [222, 223, 225, 226, 230]. The higher likelihood of organ motion, tumor regression, and change in the cervix topography oblige radiation oncologists to take great caution when applying tight treatment fields [231−237].
Brachytherapy
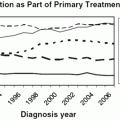
Generally, radiation therapy for cervical cancer consists of a combination of external whole-pelvic irradiation and intracavitary irradiation. The aim is to eradicate cancer in the primary tumor site, parametrial tissue, and regional lymph nodes [238]. EBRT is given initially to decrease the bulk of the tumor, providing a better geometric anatomy and allowing optimal dose delivery in intracavitary brachytherapy therapy. Brachytherapy is employed by means of uterine tandem and vaginal ovoids or ring to provide a high-radiation dose to the cervical tumor after it is partially shrunken by EBRT. The application of brachytherapy in cervical cancer patients has been proven to reduce the rate of local failure and to improve the survival rate compared with EBRT alone; 78 vs. 53 % for local control [239] and 43–87 % vs. 21.0–60.5 % for survival [240−242]. A recent Surveillance, Epidemiology, and End Results study highlighted the importance of brachytherapy in cervix cancer management. The study that was published in 2013 included over 7000 cervical cancer patients and utilized the Surveillance, Epidemiology, and End Results database using a matched cohort analysis of patients treated between 2000 and 2009. Brachytherapy used resulted in higher cancer-specific survival rates (64 vs. 52 %), and 4-year overall survival (58 vs. 46 %). Unfortunately, this study also reported a decreased utilization rate of brachytherapy between 1998 and 2009 from 83 to 58 %, respectively. This decrease in use was seen regardless of stage and histologic type [243].
LDR brachytherapy has long been used [242], but immobilization and hospitalization of patients and exposure of medical personnel to radiation have been by-products of the increasing popularity of the HDR technique in the recent years [244, 245]. It remains difficult to compare the superiority of the two methods due to poor methodology in reporting complications and loss of a large number of patients to follow-up in most studies. In a study of approximately 2000 patients, Lorvidhaya et al. [246] reported similar survival and complications at each disease stage in patients undergoing HDR and LDR brachytherapy. Still, conventional HDR brachytherapy complications, such as rectovaginal fistula, vesicovaginal fistula, ureteral stricture, and vaginal necrosis and stenosis are worrisome to many practitioners. Conventional high-dose radiation therapy for bulky tumors certainly results in a high rate of complications, such as rectovaginal fistula, vesicovaginal fistula, stricture ureter, and vaginal necrosis and stenosis [247].
Brachytherapy can be delivered with LDR, HDR, or PDR systems. LDR brachytherapy was the first modality to be traditionally used utilizing Cesium-137 based on an initial assumption of a radiobiological advantage over HDR brachytherapy [248, 249], presumably due to enhanced repair of normal tissues following LDR brachytherapy. HDR brachytherapy utilizing Iridium-192 has come into favor in many institutions because of many practical advantages that include remote after-loading that minimizes radiation exposure, use in an out-patient setting that might result in reduced cost [250], potentially better tolerance and a superior toxicity profile [251, 252], and superior treatment-plan optimization [253]. Most reports have shown similar treatment outcomes and treatment-induced adverse events in both LDR and HDR brachytherapy [244−246, 254−256]. PDR brachytherapy is theoretically considered to hold some radiobiological advantages over high dose rate (HDR) brachytherapy as each fraction comes before the complete repair of the sub-lethal cellular damage of the subsequent fraction, the tissue perceives the radiation as almost continuous, mimicking low-dose–rate (LDR) brachytherapy. Furthermore, PDR maintains the fine optimization of the dose distribution to the target volume (TV) and protect the personnel involved in the treatment from the risk of radiation exposure. Therefore, PDR hold the radiobiological advantages of LDR and the fine planning and radiation protection advantages of HDR. Although, this approach incorporates the biological advantage of LDR brachytherapy and the optimization advantage of the HDR brachytherapy, it also has many disadvantages including inpatient treatments, lack of applicator stabilization, and possibility of mechanical failure. In summary, PDR brachytherapy presents opportunities to potentially improve brachytherapy, but it also comes with detriments. Although PDR has prospered in Europe and Asia, unfortunately in the USA it has floundered because the NRC requires that a physicist and/or radiation oncologist be present throughout the treatment, which is almost impossible to accomplish in a long-treatment schedule in a hospital setting [251, 257]. Notably, many French radiation oncology centers favor the LDR and relatively recently the PDR, believing in the biological advantages of LDR and PDR and the possibility of optimizing treatment plans by controlling the source stepping time in each dwell position [258, 259].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree