Middle third of clavicle, usually right
Sternal segment usually larger, tapered, points anterosuperiorly
Acromial segment smaller, bulbous, points superomedially
Differential: birth trauma, nonunion of old fracture, cleidocranial dysostosis
Congenital pseudarthrosis of the clavicle presents as a painless, palpable, and often visible prominence in the middle one third of the clavicle, usually on the right. The lump may increase during growth, and hypermobility may result in an unattractive forward drooping of the shoulder. Parasthesias are uncommon, but thoracic outlet syndrome may develop in adulthood. Most patients present during the first 2 years of life. More than 200 cases have been reported in the literature [3]. Most are isolated abnormalities, but some result from autosomal recessive inheritance; bilateral lesions are extremely rare and may result from autosomal dominant inheritance.
The embryological development of the clavicle helps explain congenital pseudarthrosis. The clavicle, which is the first bone to ossify, develops from two ossification centers. Subsequent failure of these centers to coalesce results in congenital pseudarthrosis [4, 5]. Both vascular pulsations and bony impingement have been implicated in this lack of fusion. The almost-exclusive right-sided occurrence may result from the fact that the right subclavian artery is normally higher than the left. It must therefore pass through a narrow gap between the first rib and midclavicle, where it can apply pressure on the developing bone [6]. Most reported cases of congenital pseudarthrosis on the left occur in patients with dextrocardia, supporting the theory that vascular pulsations contribute to the lesion. Furthermore, cervical ribs or superior extension of the thorax and first rib are often present, exacerbating impingement. Bilateral lesions are rare, with only nine reported cases [7]. They are associated with bilateral high subclavian arteries, cervical ribs, and/or high, vertically oriented upper ribs [8].
Histological studies of resected specimens have shown that both ends of bone are covered with hyaline cartilage caps composed of columnar chondrocytes, resembling normal growth cartilage. Fibrocartilage connects these hyaline caps [4, 5].
Treatment is generally surgical, although there is a high rate of failure and complications [4, 7, 9]. Surgery usually consists of resection, bone graft placement, and internal fixation [3, 7, 10].
Imaging
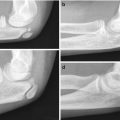
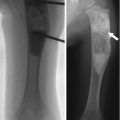
On radiographs, absence of bone in the midportion of the clavicle is obvious in young children, but the configuration varies [10]. The classic appearance consists of a large sternal portion that protrudes anteriorly and superiorly and a smaller more caudally positioned acromial segment that points superiorly and medially (Fig. 5.1). Typically the lateral end of the sternal segment is tapered, and the medial end of the acromial fragment is expanded or bulbous [11]. However, both the configuration of and the relationship between the ends of the two clavicular fragments may vary (Fig. 5.2) [10]. There is no callus or periosteal reaction. Radiographs are almost always diagnostic, but very rarely—if the pseudarthrosis is hidden behind the apex of the deformity and if the history is atypical—additional imaging such as computed tomography with three-dimensional reconstruction (3D-CT) may be necessary to exclude such lesions as Ewing sarcoma [12].
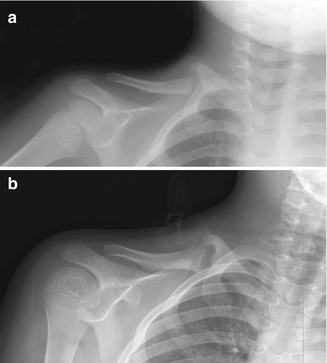
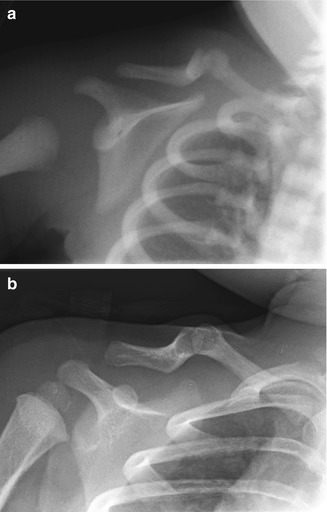

Fig. 5.1
Congenital pseudarthrosis of the clavicle in a 3-year-old who presented with a palpable mass. (a) At presentation, there are typical smooth, tapered contours without evident callus formation. (b) 5 years later, nonunion persists, although the medial fragment abuts the lateral fragment (Images copyright Shriners Hospital for Children Northern California)

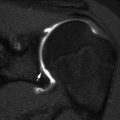
Fig. 5.2
Congenital pseudarthrosis of the clavicle in a newborn, discovered incidentally during evaluation for respiratory distress. (a) Right clavicular defect with ends that are smoothly contoured, tapered, and sclerotic. The medial fragment is elevated by pull of the sternocleidomastoid muscle. (b) One year later, the ends are more closely apposed and remain smooth (Images copyright Shriners Hospital for Children Northern California)
Differential Diagnosis
If a radiograph is obtained in the newborn, the typical configuration often allows differentiation from birth fracture; lack of subsequent callus formation helps in equivocal cases. A postnatal fracture is usually differentiated by typical history and development of callus [10]. If there is nonunion of an old fracture, both ends are usually attenuated [8]. Differentiation from cleidocranial dysostosis is more difficult, since the appearance can be identical—in 40 cases of cleidocranial dysostosis, 17 of the clavicular lesions were unilateral and affected only the right side [13]. A careful search for cranial sutural and fontanelle defects, pubic symphysis diastasis, and the other characteristic spine and hand abnormalities should be made, especially if there is a family history of similar abnormalities in this autosomal dominant dysplasia.
4 Congenital Undescended Scapula (Sprengel Deformity) (Box 5.2)
Box 5.2: Sprengel Deformity (Congenital Undescended Scapula)
Klippel-Feil associated in 20 % |
Omovertebral bone in 50 % |
Scapula tilted, lifted, medially displaced |
Acromion elongated |
Patients with Sprengel deformity typically present with fullness at the base of the neck and loss of shoulder motion; average age at diagnosis is 3 years [14]. Three times more common in girls, it is usually on the left but occasionally bilateral or on the right. Almost all patients have accompanying anomalies. Scoliosis occurs in 39 %, usually convex on the same side as the scapular elevation. Accessory, absent, fused, or bifid ribs are seen in 25 %. Twenty percent have developmental anomalies of the cervical spine, such as hemivertebrae and vertebral fusion (constituting the Klippel-Feil anomaly) (Fig. 5.3). Meningocele or dysraphism occur in 7 %, and genitourinary abnormalities are common [15]. The adjacent musculature is typically hypoplastic and fibrotic [16].
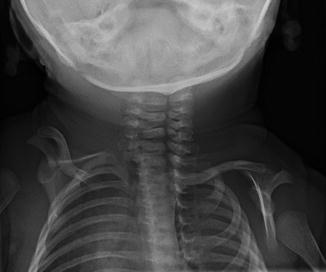

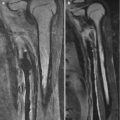
Fig. 5.3
Sprengel deformity on the right in a 3-week-old girl. The scapula is elevated, angulated, and medially displaced
Sprengel deformity occurs when the scapular anlage fails to descend from the C4–C5 level to its normal position below T3. A physical connection between the medial angle or edge of the scapula and the lamina, spinous, or transverse process of the spine from C4–T1 is common [17], but it is uncertain whether this tethering causes or results from the anomaly. The site of insertion appears to affect scapular shape [18]. A bony connection between the scapula and occiput has rarely been described [19].
A fibrous or cartilaginous connection to the spine is common. Osseous condensation occurs in up to 50 % of patients and is termed the omovertebral bone [15, 19]. This bone may represent a rudimentary cervical rib or the homologue of the suprascapular bone seen in some lower vertebrates [20].
Children with mild deformity can be treated with physical therapy to maintain range of motion and are generally followed until skeletal maturity. For more severe deformities, various surgical procedures have been developed, including repositioning and osteotomy of the scapula and resection of the omovertebral bone [1]. These procedures are thought to be most successful before 6 years of age [14].
Imaging
On radiographs, the extent of scapular elevation and medial displacement varies; often the scapula is tilted with the medial angle high but the glenoid and shoulder at near-normal level. The scapula appears small but is actually larger than normal [18]. Its shape is infantile, with an increased horizontal and decreased vertical length—the latter may be partly due to projectional foreshortening, since the scapula is often angulated forward (Fig. 5.4). The acromion may be elongated and carried downward, and there is generally a rotational component to the deformity as well. Minor changes in projection can dramatically alter the apparent position of the scapula [14], and overlapping structures limit visualization. 3D-CT provides more accurate information and can assist with surgical planning (Fig. 5.5) [18]. Either computed tomography (CT) or magnetic resonance imaging (MRI) allows improved identification of the omovertebral bone [21].
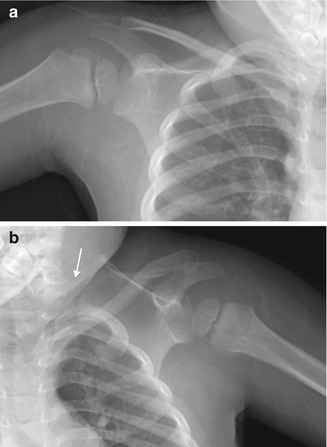
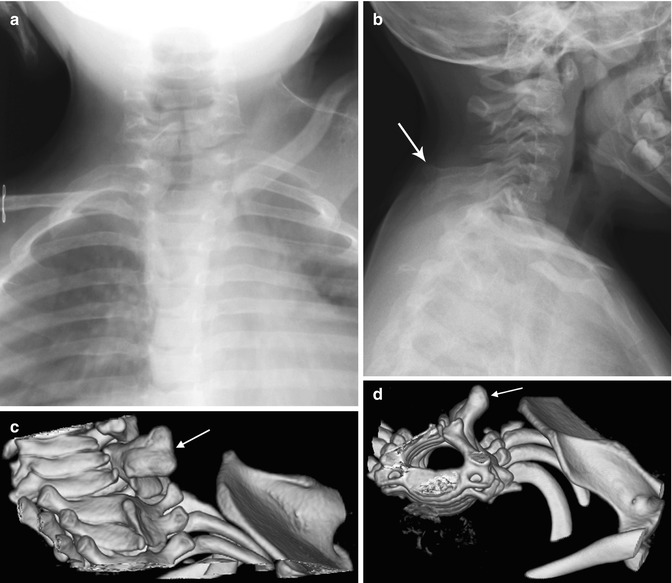

Fig. 5.4
Sprengel deformity on the left. (a) The right shoulder is normal. (b) The left scapula is elevated, rotated, and medially displaced. The omovertebral bone is difficult to see (arrow), extending laterally from the lower cervical spine (Images copyright Shriners Hospital for Children Northern California)

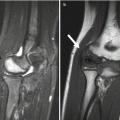
Fig. 5.5
Sprengel deformity in a 15-month-old girl. (a) The left scapula is elevated, rotated, and medially displaced. (b) Note bulbous fusion of spinous processes of the lower cervical spine. (c, d) 3D-reconstructed CT images suggest omovertebral bone (arrows) extending toward the superomedial scapula, probably with an intervening fibrous segment
5 Glenohumeral Dysplasia (Box 5.3)
Box 5.3: Glenohumeral Dysplasia
US up to 12 months | Delineates cartilage, quantifies subluxation |
Radiography | Humeral head—small, flat,and deformed |
Scapula—elevated and rotated out with small glenoid | |
Acromion process—elongated and hooked | |
MRI | Glenoid fossa—normal, flat, or biconcave |
The brachial plexus is injured during obstetric delivery in 2.3–3.3 per 1,000 live births [22]. Several identifiable syndromes result from these nerve injuries. In 80 % of patients, C5 and C6 branches are affected and a classic upper arm (Erb’s) palsy results—paralysis of the external rotators and tightness of the internal rotators result in loss of both active and passive external rotation. The arm hangs in internal rotation, the forearm is pronated, and the wrist flexed—the “waiters tip” deformity. The upper arm may appear foreshortened if there is posterior dislocation at the glenohumeral joint, which causes telescoping of the soft tissues and extra skinfolds. There may be fullness of the posterior aspect of the shoulder. Persistent muscle imbalance results in progressive posterior shoulder dislocation; this has usually been diagnosed after the age of 1 year, but studies have shown that it can develop as early as 4 months in 7–8 % of patients with brachial plexus birth injury [22, 23]. The phrenic nerve, predominantly C4 but with contributions from C5, may also be involved.
When the whole arm is involved (C5–Tl lesions of Duchenne-Erb-Klumpke), flaccidity is obvious and there may be an associated Horner’s syndrome. However, muscle damage tends to be more balanced, resulting in less glenohumeral dysplasia [24, 25].
The brachial plexus is usually injured in the course of breech deliveries. Birth weight over 4,000 g increases the risk of brachial plexus injury by a factor of 2.5 [26]. Other risk factors include multiparous pregnancies, shoulder dystocia in vertex deliveries, and vacuum-assisted deliveries [27]. Reversible hemorrhage and edema may result from nerve plexus traction, and thus gradual near-complete recovery occurs in 93–95 % [22, 26]. When partial or total nerve transection occurs, full recovery is unlikely; injury is irreversible if the nerve root is torn from the spinal canal injury [27]. With progressive neuromuscular imbalance, the cartilage of the glenoid and humerus becomes increasingly dysplastic, as with developmental dysplasia of the hip. Eventually, there is bony remodeling.
Treatment is intended to restore range of motion and establish a stable glenohumeral relationship in order to maximize the functional potential of the affected arm. There is some evidence that glenoid remodeling is facilitated by rebalancing musculature, although it is unclear how much glenohumeral dysplasia can be reversed and at what age [28, 29]. Botulinum toxin injection, splinting, and stretching are useful initial treatment for children under 1 year of age, and physical therapy is often continued throughout childhood. In those less than 6–9 months of age, microsurgery may be employed to reconstruct the brachial plexus. Tendon transfers and capsular releases (either open or arthroscopic) are useful in children over 1 year of age who develop glenohumeral dysplasia and/or persistent limited external rotation. Humeral derotational osteotomy may be performed in older children with advanced dysplasia [30]. Other surgical procedures including tendon transfers and osteotomies are used throughout the upper extremity to address dysfunction of the elbow, wrist, and hand as needed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree