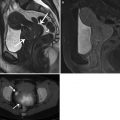
Fig. 10.1
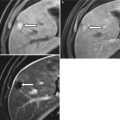
Coronal reconstruction shows an example of ideal CTE technique. Note good distension of the small bowel loops with VoLumen, with optimal mucosal enhancement. Neutral oral contrast agents (VoLumen) with a density of 0–30 HU were adopted because they allow improved visualization of bowel wall enhancement, mural features, enhancing mucosal and submucosal masses, vascular malformations and contrast extravasation into the bowel lumen. Neutral enteric contrast materials include Volumen: a commercially available neutral agent containing barium sulfate suspension (VoLumen; Bracco Diagnostics, Princeton, NJ), methylcellulose, polyethylene glycol (PEG) solution, or water. The very low-density barium and sorbitol contrast solution typically achieves better small bowel distension compared with water and methylcellulose
Neutral oral contrast agents (VoLumen) with a density of 0–30 HU were adopted because they allow improved visualization of bowel wall enhancement, mural features, enhancing mucosal and submucosal masses, vascular malformations, and contrast extravasation into the bowel lumen. Neutral enteric contrast materials include VoLumen: a commercially available neutral agent containing barium sulfate suspension (VoLumen; Bracco Diagnostics, Princeton, NJ), methylcellulose, polyethylene glycol (PEG) solution, or water. The very low-density barium and sorbitol contrast solution typically achieves better small bowel distension compared with water and methylcellulose.
Administration of large volumes (1,350 ml) of enteric contrast agent is necessary for achieving optimal small bowel distension. Patients are NPO for 6 h prior to imaging. As per the current regimen, a total of 1.35 L of VoLumen is ingested over 1 h: 450 mL at 60 min, 450 mL at 40 min, and 225 mL at 20 min, with the last 225 ml bolus immediately before CTE imaging [4]. While used by some institutions, the efficacy of glucagon to improve small bowel distention in CTE has not been validated.
Rapid intravenous administration of contrast material (4 mL/s) is necessary for adequate visualization of the bowel wall with optimal mucosal enhancement (referred to as the enteric phase). Single-phase imaging in the venous phase after the start of IV contrast administration is sufficient to evaluate inflammatory bowel disease and small bowel tumors. In addition, the use of advanced reconstruction techniques, such as iterative reconstruction, enables reduction in the radiation dose [4]. A multiphasic study may be performed for gastrointestinal bleeding and mesenteric ischemia with acquisition of pre-contrast, arterial phase (20 s delay or timed via automated bolus triggering such as smartprep), and delayed phase (90 s delay or longer delays for GI bleed studies of course can be used also). Pre-contrast images help to differentiate incidental hyperdense material in the bowel lumen from abnormal enhancement. At most institutions, however, the multiphasic CT studies are performed after negative endoscopy and colonoscopy studies due to radiation concerns. Multiphasic imaging evaluates vascular malformations (demonstrating associated early draining vein) and mesenteric arteries and veins in the setting of ischemia [5].
It is important to reconstruct images in coronal and sagittal planes, as well as coronal maximum intensity projections. A combination of multiple planes may be necessary to detect all the manifestations of inflammatory bowel disease and identify a tumor or source of gastrointestinal bleeding on CTE. Overall, tailoring the imaging technique according to the prevalent clinical condition is extremely critical (as illustrated in Algorithm 10.1).
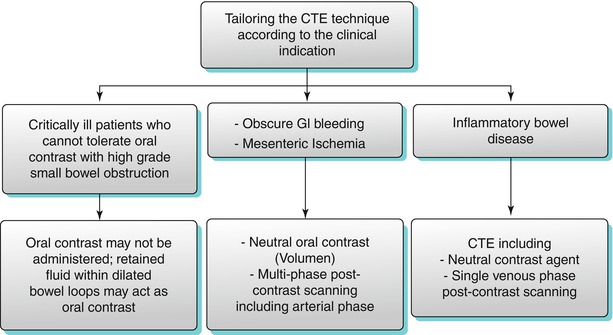

Algorithm 10.1
Tailoring the CTE technique according to the clinical indication
Magnetic Resonance Imaging
Until recently, MRI played a limited role in bowel imaging due to long scan times and motion artifacts due to bowel peristalsis. The clinical utility of MR in assessing small bowel pathology has rapidly improved bowel imaging because of the development of fast breath-hold sequences (eliminating motion artifacts) and availability of suitable luminal contrast agents (allowing optimal distension) [6]. Also, increasing awareness of radiation dosage from CT exposure has furthered MR enterography with neutral enteric contrast (such as VoLumen) as an emerging technique to evaluate for small bowel pathology. MR has several inherent advantages over CT including lack of radiation exposure, eliminating the need for iodinated contrast material, and outstanding soft tissue contrast. The lack of radiation exposure is particularly important in pregnant or pediatric patients or in patients undergoing serial follow-up examinations [6]. Disadvantages of MRI include higher costs and longer exam times compared to CT and known contraindications to MR such as pacemakers. Gadolinium administration may be contraindicated in patients with renal insufficiency due to nephrogenic systemic fibrosis (NSF).
Some authors propose that small bowel can be adequately assessed utilizing heavily T2-weighted sequences and intrinsic intestinal fluid, without the need for oral contrast ingestion. The use of several enteric contrast agents has been described for MRE such as VoLumen [6]. These agents delay the absorption of water in the intestine achieving adequate enteric distention. Most MRE protocols require that the patient be NPO for 6 h prior to the procedure. Fasting decreases the amount of enteric food residue and debris, which may be otherwise mistaken for masses or polyps.
Important factors in enteric contrast selection include the ability to achieve homogeneous bowel opacification and bowel distention, high contrast resolution between the lumen and bowel wall, no significant adverse effects, low artifact potential, and low cost. Thus, agents poorly absorbed by the bowel are preferred to achieve adequate bowel distention. Approximately, 1,350 mL of VoLumen is administered over 1 h before the examination. VoLumen can be divided into 450 ml 60 min, 450 ml 40 min, 225 ml 20 min, and 225 ml 10 min before the examination. Ingesting the contrast solution over the allocated time period is important to achieve homogeneous bowel opacification and optimal bowel distention. The patients are also asked to drink another 200 ml of contrast material just before imaging to achieve opacification of the stomach and duodenum. An IV injection of 1 mg of glucagon is administered to minimize bowel peristalsis [6].
MRE can be performed using single breath-hold ultrafast MR sequences based on steady-state precession. These sequences include true fast imaging with steady-state precession (FISP), balanced fast field-echo, or fast imaging employing steady-state precession depending on the machine vendor. These sequences provide high contrast between the bowel wall, lumen, and mesentery, and at the same time, these are relatively insensitive to motion artifacts. Disadvantage associated with these MR sequences is a black boundary artifact along the bowel wall, which can mask small lesions. Fat suppression technique helps reduce the effect of this artifact. T2-weighted sequences, based on RARE, also known as SSFSE or HASTE, produce high contrast between the lumen and the bowel wall and thus better demonstrate ulcers, mural edema, and fluid collections. Disadvantages of these sequences include susceptibility to flow voids. Contrast-enhanced images can be acquired using either 2D or 3D fat-saturated gradient echo T1-weighted sequences [6].
An advantage of MR imaging versus CTE is that MRE may allow differentiation between the various kinds of bowel wall changes that may produce nonspecific low attenuation at CTE. For example, on T2-weighted fat-saturated MR images, mucosal or submucosal edema is depicted as a layer of high signal intensity accompanied by bowel wall thickening in an otherwise normally hypointense bowel wall. This suggests active disease. In long-standing disease, submucosal fat deposition is demonstrated as high signal intensity on T1-weighted images. MR imaging can also distinguish the bowel wall edema from fibrotic wall thickening, which usually has low signal intensity on both T1- and T2-weighted images [7].
Capsule Endoscopy
Capsule endoscopy relies on a swallowed video capsule, which allows noninvasive endoscopic assessment of the entire small bowel mucosa, without the need for sedation. This technique has relative limitations, which include limited evaluation of the entire length of the small bowel. Capsule endoscopy is primarily used to assess mucosal-based pathologies, including obscure gastrointestinal hemorrhage, tumors, and ulcers. However, this technique is not ideal to localize and definitively treat the lesions. Endoscopic capsule can be retained proximal to inflamed or fibrotic strictures. CTE can be used in the evaluation of small bowel disease before the administration of the capsule endoscopy to avoid this complication. The relative advantages and disadvantages of capsule endoscopy versus CTE are listed below (Algorithm 10.2).
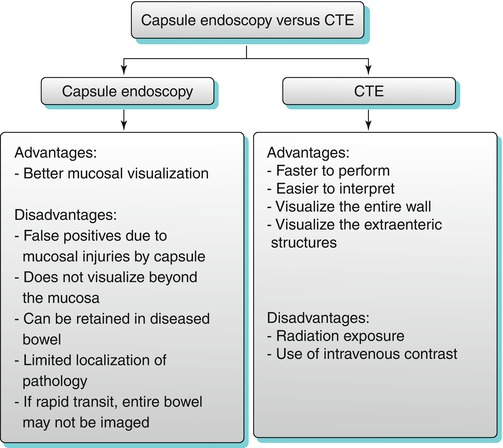

Algorithm 10.2
Capsule endoscopy versus CTE
Approach to Small Bowel Pathologies: Small Bowel Wall Thickening
Bowel wall thickening may or may not be associated with obstruction and proximal bowel dilatation. In cases of wall thickening, the radiologist must evaluate the following features in relation to the involved bowel loop: location of involvement, length of involvement, concentric or eccentric wall involvement, wall enhancement, location of the lesion in the wall, and extraluminal findings involving the mesentery and vasculature.
Algorithmic Approach to Abnormal Small Bowel Wall Thickening
An algorithmic approach for diagnosis of various small bowel diseases is suggested which include small bowel wall thickening (Algorithm 10.3), location of the thickening (Algorithm 10.4), length (Algorithm 10.5), pattern of enhancement (Algorithm 10.6), epicenter of the lesion in relation to the bowel (Algorithm 10.7), as well as extraluminal manifestations (Algorithm 10.8).
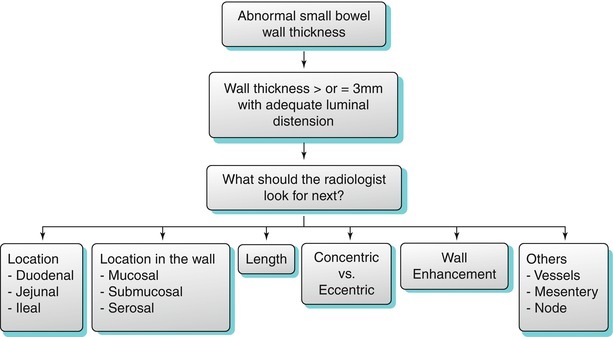

Algorithm 10.3
Algorithmic approach to small bowel wall thickening

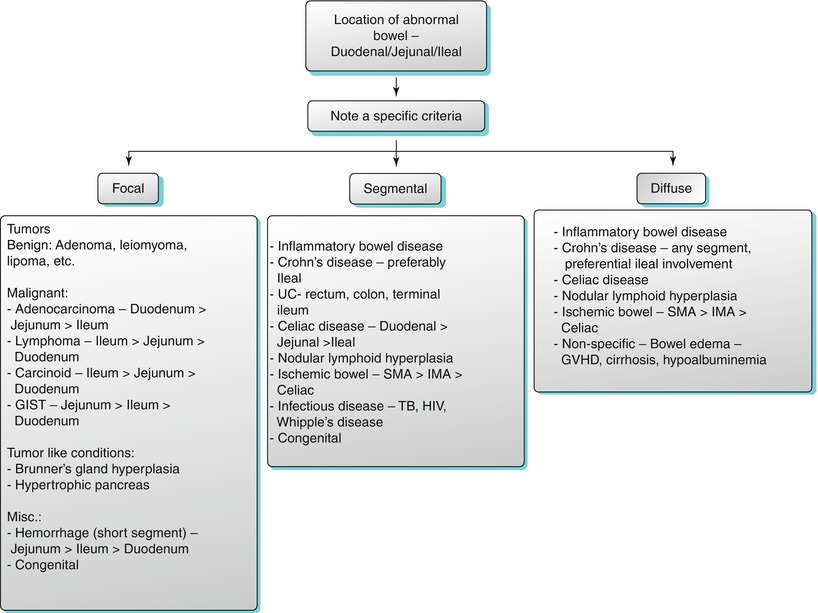
Algorithm 10.4
Location of abnormal small bowel thickening

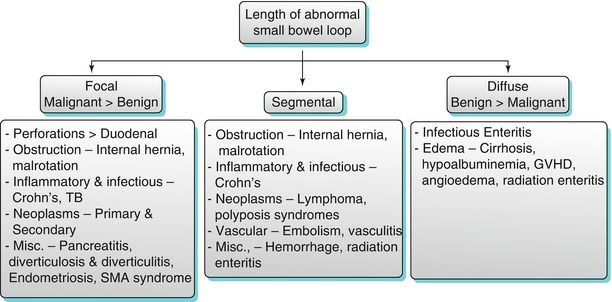
Algorithm 10.5
Length of abnormal small bowel thickening

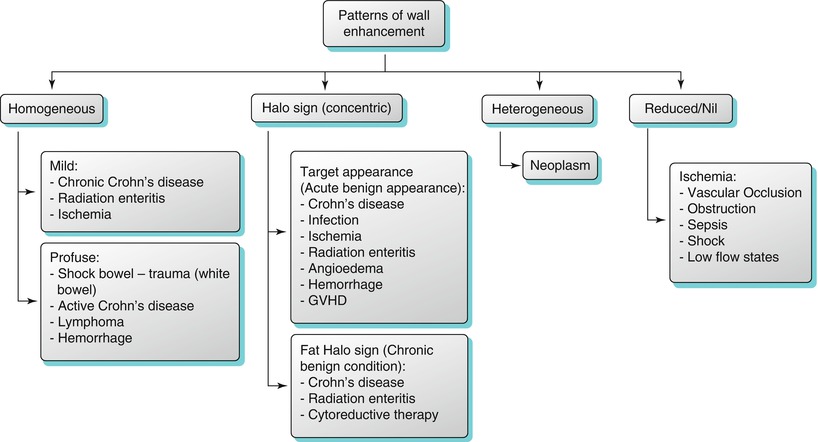
Algorithm 10.6
Patterns of bowel wall enhancement

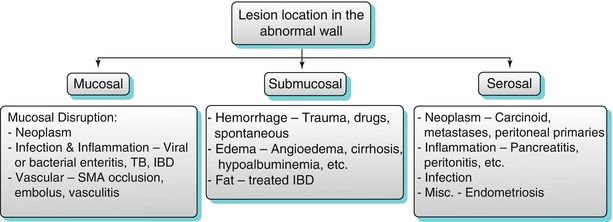
Algorithm 10.7
Epicenter of lesion in relation to the bowel wall

Algorithm 10.8
Extra-bowel imaging findings related with bowel wall thickening
Small Bowel Obstruction and Dilatation
The normal small bowel diameter should be less than 3 cm. If the luminal diameter is greater than 3 cm with no transition point, a small bowel ileus may be present. If the small bowel lumen is greater than 3 cm in diameter, air fluid levels are present, and if there is a transition point (with decompressed distal small bowel loops) (Fig. 10.2 and Algorithm 10.9), small bowel obstruction from various etiologies must be considered (Algorithm 10.12). Small bowel obstruction can be termed as simple or closed loop obstruction and may be partial (low grade) or complete (high grade) (Algorithm 10.10). Small bowel obstruction with intact blood supply is termed simple (Algorithm 10.11). In partial obstruction, there is narrowed bowel lumen with conserved passage of gas and intestinal contents distally. In complete obstruction, there is total luminal occlusion with paucity of gas and fecal material in colon or decompressed distal small bowel. In closed loop obstruction, the bowel segments occluded at two points are usually adjacent to each other, and this type of bowel obstruction is more prone to ischemia potentially resulting in bowel necrosis, sepsis, peritonitis, and death. The involved small bowel loop in a closed loop obstruction is usually fluid filled and may assume a coffee bean (U shape) or C shape on CT imaging (Figs. 10.3 and 10.7). Moderate to severe small bowel obstruction may be confirmed by the presence of small bowel feces sign (Fig. 10.4); however, this is neither sensitive nor specific for the diagnosis. The small bowel feces sign is defined as the presence of gas and particulate material within a dilated small bowel loop that simulates the appearance of feces and is most likely caused by stasis within the obstructed loop, allowing more time for fluid absorption across the bowel wall and accumulation of undigested food particles. Strangulated obstruction involves small bowel obstruction with evidence of bowel ischemia. Strangulation may occur in a setting of closed loop obstruction, which on CECT may appear as thickened bowel wall, hemorrhage in the bowel wall and mesentery, non-enhancing bowel wall, engorged mesenteric vessels, and pneumatosis (Fig. 10.5). Occasionally, small bowel dilatation may result in a setting of a large bowel mass associated with a patulous ileocecal valve (Fig. 10.6).
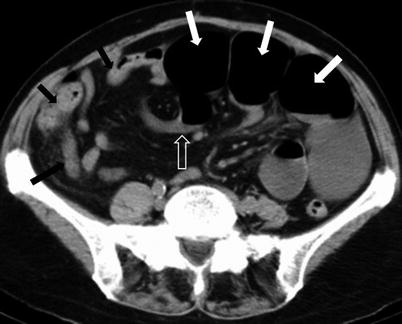
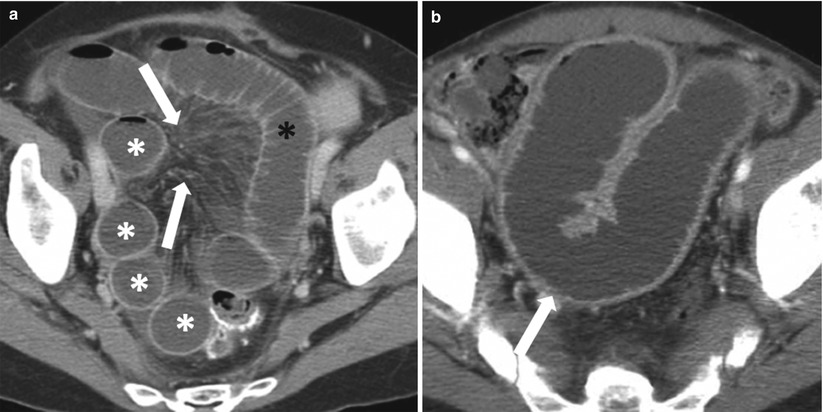
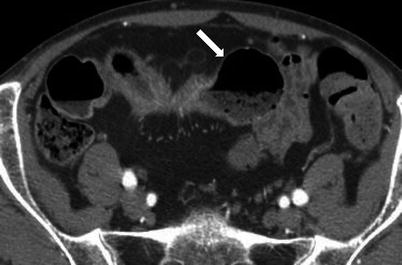
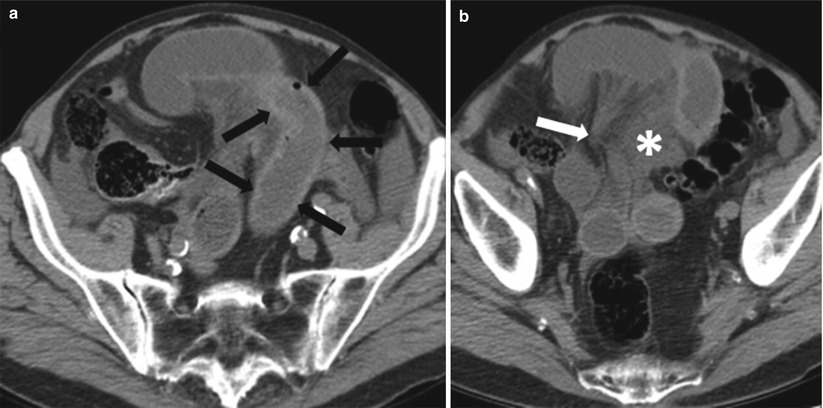
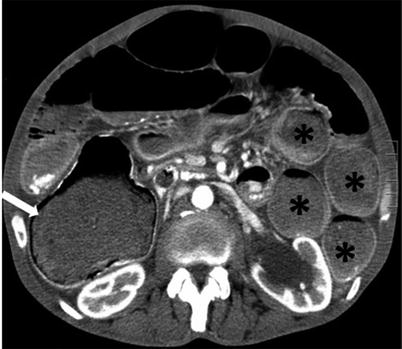
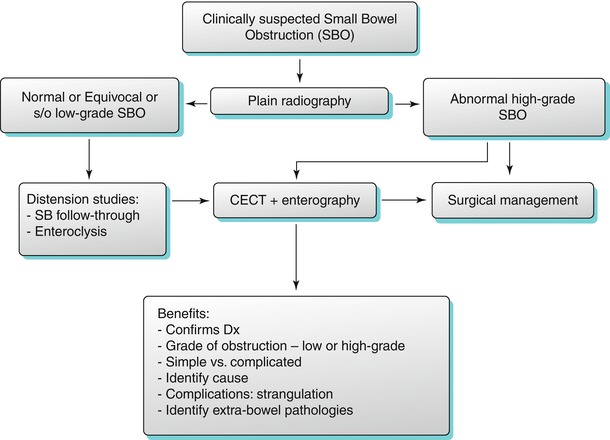
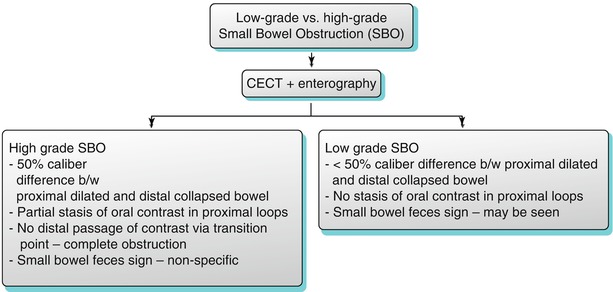
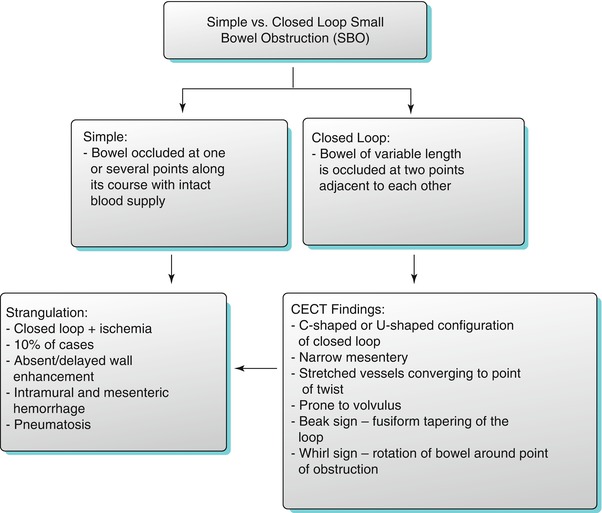
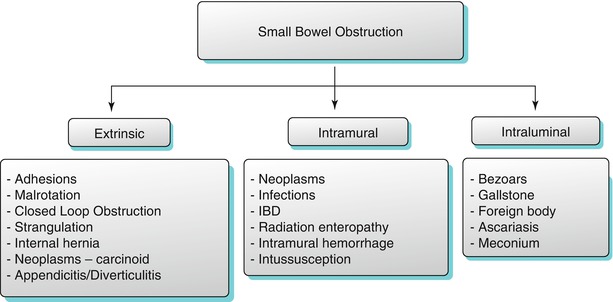

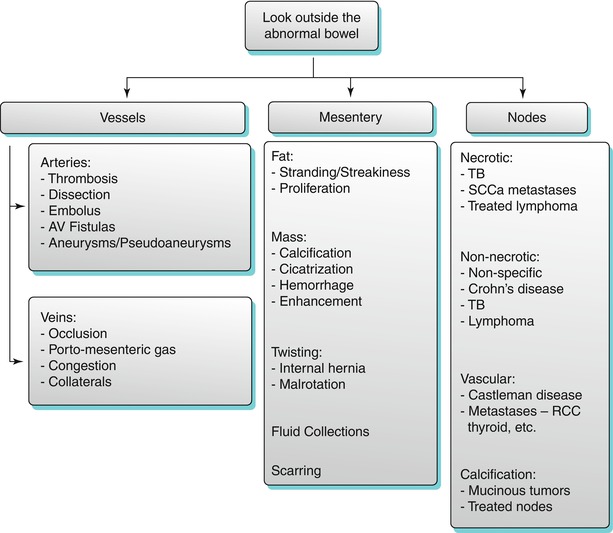
Fig. 10.2
Small bowel obstruction with a transition zone. Axial contrast-enhanced CT obtained during the venous phase shows dilated fluid and gas-filled small bowel loops aggregated in the left side of the abdomen (white arrows), with abrupt narrowing (transition point – transparent arrow) and collapse of the distal bowel loops (black arrows)

Fig. 10.3
Closed loop obstruction. Axial contrast-enhanced CT of the pelvis (a) obtained during the venous phase, shows an in-plane C-shaped loop on the left (black asterisk), with multiple out-of-plane radially arranged loops to the right (white asterisks), and convergence of the mesenteric vessels to the point of narrowing (white arrows). Contrast-enhanced axial image of the pelvis (b) obtained during the venous phase, shows U-shaped dilated small bowel loop (white arrow) secondary to closed loop obstruction

Fig. 10.4
Small bowel feces sign. Axial contrast-enhanced CT image of the pelvis obtained during the venous phase shows a dilated small bowel loop with entrapped air and particulate matter (white arrow)

Fig. 10.5
Strangulation of small bowel. Axial contrast-enhanced CT images (a, b) of the pelvis obtained during the venous phase show a closed loop obstruction, with hyperdense wall thickening of the C-shaped bowel loop due to hemorrhage (black arrows), mesenteric vessel convergence (white arrow) and mesenteric edema (white asterisk)

Fig. 10.6
Cecal carcinoma with patulous ileocecal valve and small bowel dilatation. Axial contrast-enhanced CT image of the abdomen obtained during the arterial phase shows an eccentric cecal mass (white arrow), with dilated small bowel loops (black asterisks) due to a patulous ileocecal valve

Algorithm 10.9
Algorithm for radiological evaluation of small bowel obstruction

Algorithm 10.10
Imaging algorithm for differentiating low-grade versus high-grade small bowel obstruction

Algorithm 10.11
Imaging algorithm for differentiating simple versus closed loop small bowel obstruction

Fig. 10.7
Closed loop small bowel obstruction. Sagittal reconstructed and axial contrast enhanced CT images (a and b) demonstrate the “beak sign” (arrows in a and b) associated C/U shape of the dilated small bowel loops (a, b). Axial contrast enhanced CT in a different patient (c) demonstrates “whirl sign” (white arrow) with rotation of the bowel loop around point of obstruction (c). Axial contrast enhanced CT images in two different patients (d, e) demonstrate multiple dilated bowel loops (black asterisks) with radial distribution of vessels

Algorithm 10.12
Etiological classification of small bowel obstruction
Pathological Classification of Small Bowel Pathologies
Anomalies of Rotation and Fixation
Congenital rotation anomalies involving the midgut often present within the first month of life. However, few patients present during the later years or remain asymptomatic for life. Malrotation is defined as any deviation from the normal 270° counterclockwise rotation of the midgut during embryologic development [8]. These anomalies are classified as either incomplete rotation or nonrotation [9]. Malrotation results in malposition of the bowel and is often associated with mesenteric malfixation as noted by the presence of a narrow pedicle of attachment, which predisposes to midgut volvulus. Rotation anomalies may occur in combination with situs abnormalities in approximately 70 % of the cases [10].
On CECT, malrotation appears as an abnormal duodenojejunal junction that fails to cross the midline and lies below the level of the duodenal bulb and with malposition of the right colon (Fig. 10.8). However, in 20 % of patients, the cecum may be located in a normal location, and in such cases, a quiescent malrotation may be missed [9]. Deviation of the normal relationship between the SMA and SMV is a useful indicator of malrotation [11], with a vertical relation or left–right inversion seen in most cases [12]. Importantly, an abnormal SMA–SMV relationship in isolation is not entirely diagnostic of malrotation [12]. An additional finding seen in cases of malrotation is underdevelopment of the uncinate process of pancreas.
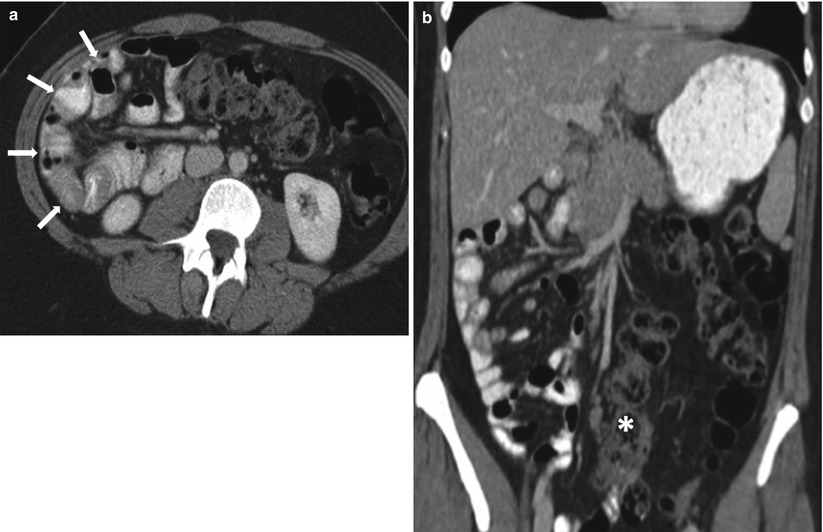

Fig. 10.8
Malrotation. Axial contrast-enhanced (a) and coronal reconstructed (b) images of the abdomen obtained in the venous phase show small bowel loops occupying the right side of abdomen (white arrows) with malposition of the ascending colon (white asterisk)
Malrotation may be complicated by midgut volvulus secondary to a narrow mesenteric attachment, which results in clockwise twisting of the bowel around the SMA axis. On CECT, features of volvulus include a corkscrew appearance of the proximal small bowel, duodenal obstruction, congestion of the mesenteric vasculature, and mesenteric ischemia. Another complication of malrotation is internal hernia, caused by abnormal peritoneal Ladd bands.
Duplication Anomalies
Enteric duplication cysts are an uncommon congenital abnormality, which typically occur on the mesenteric side of the gut. The small intestine is the most common site of involvement, with the ileum being the preferred site. Duplication cysts usually manifest in the first year of life, although late manifestation in older patients is reported [13]. Duplication cysts may be complicated by intussusception, bowel obstruction, volvulus, perforation, and malignancy. Duplication cysts may be associated with vertebral or urogenital malformations [14].
On USG, these cysts appear as fluid-filled sacs with an echogenic inner mucosal layer surrounded by a hypoechoic outer muscular layer of variable thickness, which is further surrounded by an outer echogenic serosa. On CECT, a duplication cyst appears as a non-enhancing cystic mass, which may be complicated by the presence of intracystic hemorrhage or proteinaceous material (Fig. 10.9) [15]. Occasionally gastric mucosa may be present in a duplication cyst, which may be identified using a technetium-99m sodium pertechnetate study.
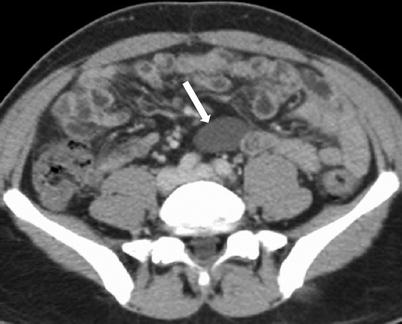

Fig. 10.9
Enteric duplication cyst. Axial contrast-enhanced CT obtained in venous phase shows non-enhancing cystic mass along the mesenteric side of ileal loop (arrow)
Meckel’s Diverticulum
Meckel’s diverticulum results from an incomplete involution of the omphalomesenteric duct [16] and represents the most common congenital anomaly of the gastrointestinal tract, with an approximate incidence of 2–3 % [17]. It is usually located within 2 ft from the ileocecal valve. This is a true diverticulum occurring along the antimesenteric border of the distal ileum (Fig. 10.10) and frequently contains heterotopic gastric or pancreatic mucosa. The diverticulum may connect with the umbilicus via a fibrous band.
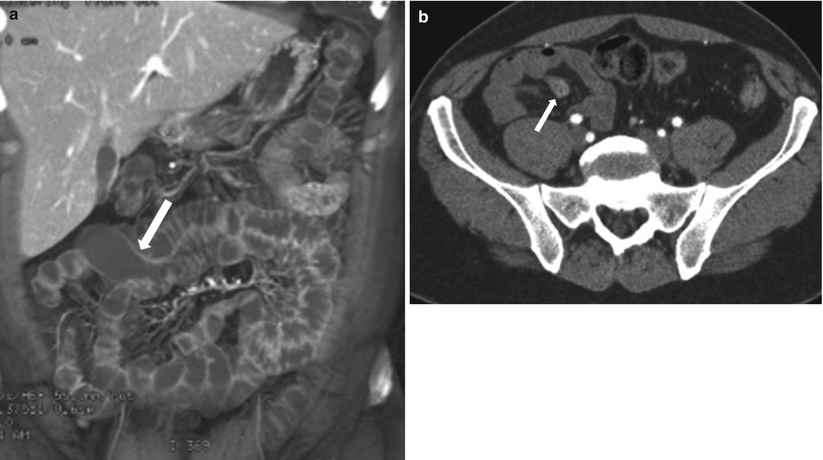

Fig. 10.10
Meckel’s diverticulum. Axial coronal reconstructed CT image (a) show a flask shaped fluid-filled blind ended outpouching (arrow) off ileal loop consistent with incidentally noted Meckel’s diverticulum. Axial contrast enhanced CT (b) in a different patient, shows Meckel’s diverticulum (arrow) with enhancing mucosa in this patient presenting with obscure gastrointestinal bleeding
On CECT, Meckel’s diverticulum appears as a saccular, blind-ending structure along the antimesenteric border of the distal ileum, with a triradiate fold pattern converging to the ileum. Complications associated with Meckel’s diverticulum include enteroliths, hemorrhage, inflammation (diverticulitis), obstruction, inversion that may produce obstruction or act as a lead point for intussusception, and rarely neoplasms.
Inflammatory Bowel Disease
Crohn’s Disease
Crohn’s disease typically involves the small bowel, with dominant involvement of the terminal ileum. The pattern of involvement is typically transmural with characteristic skip lesions and often penetrating disease in the form of enteric fistulae or abscesses [18]. Conventional oral and intravenous contrast-enhanced CT does not precisely identify inflammatory small bowel disease. In such a setting, CT enterography is an invaluable technique to diagnose inflammatory bowel disease as it exquisitely delineates the bowel wall and is useful in differentiating active inflammatory strictures from fibrotic strictures. This information is critical, as active inflammation is likely to be treated medically, whereas fibrotic strictures are likely to have a surgical outcome [19, 20]. Capsule endoscopy is another technique to evaluate the inflammatory small bowel disease; however, it is more useful in delineating mucosal-based pathologies rather than mural pathologies [21]. Capsule endoscopy is reserved for patients with a strong clinical suspicion for Crohn’s disease with negative CT enterography results. However, small bowel capsule endoscopy is contraindicated in the presence of inflamed or fibrotic strictures with a potential for impaction leading to bowel obstruction (Fig. 10.11).
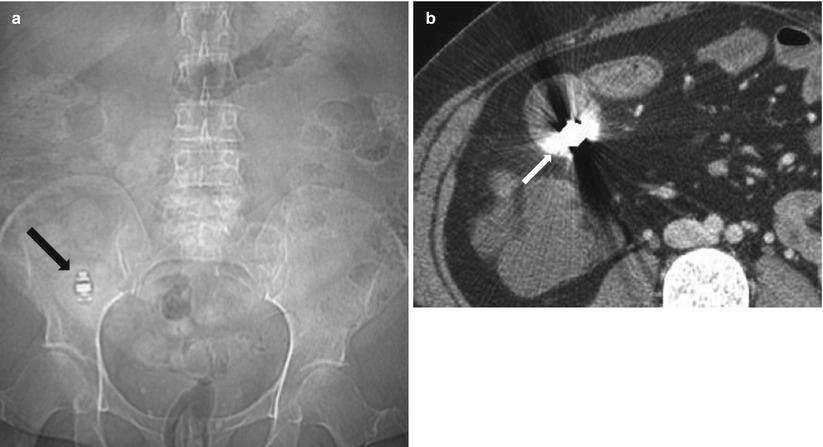

Fig. 10.11
Retained capsule in Crohn’s disease. Plain radiograph (a) and axial contrast enhanced CT enterography (b) demonstrate impaction of a capsule (arrow in a and b) within the lumen of thick walled ileum
Characteristic CT enterographic findings of active Crohn’s disease include bowel wall thickening, segmental mucosal hyperenhancement, mural stratification, perienteric mesenteric fat stranding, and engorged vasa recta [22, 23]. Bowel wall thickening is typically eccentric, with involvement of the mesenteric border, and occasionally associated with pseudosacculations, sinuses, fistulae, or intramural/extraenteric abscesses. Segmental mucosal hyperenhancement is the most sensitive feature of active disease [24] and typically refers to increased attenuation of inflamed mucosa relative to nearby normal-appearing loops. If the bowel loops are collapsed, secondary signs of mural inflammation need to be elicited prior to making the diagnosis. Mural stratification represents visualization of a trilaminar appearance of the bowel wall, with intense enhancement of the mucosa and serosal layers of the involved bowel, interspersed by a hypoattenuating edematous submucosal layer (Fig. 10.12). Skip lesions are pathognomonic of Crohn’s disease (Fig. 10.13). Absence of the trilaminar pattern but presence of a homogeneously enhancing wall suggests a fixed fibrotic stricture (Fig. 10.14). Engorged vasa recta, also called the comb sign (Fig. 10.15) [25, 26], appears as vessels penetrating the bowel wall perpendicularly and is a characteristic sign of active inflammation. Comb sign is known to correlate with the disease severity [27].
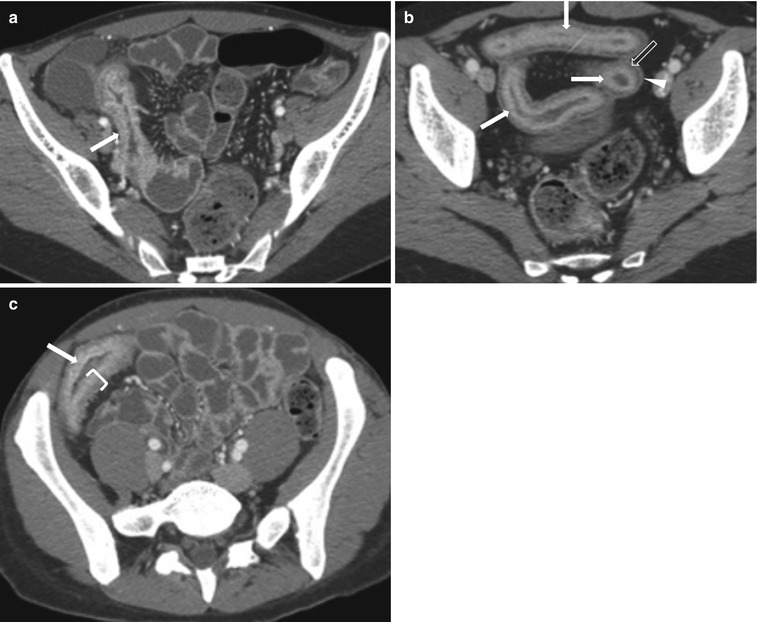
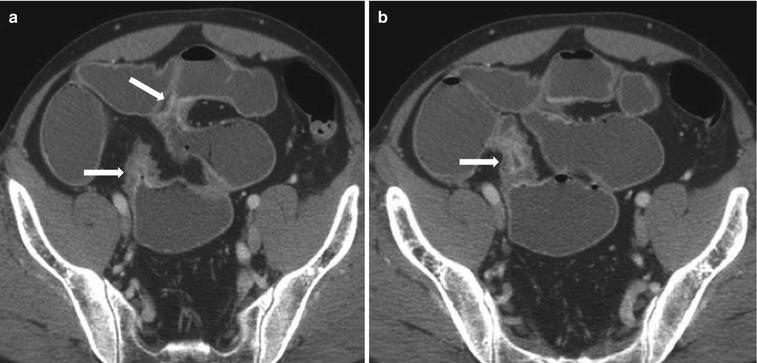
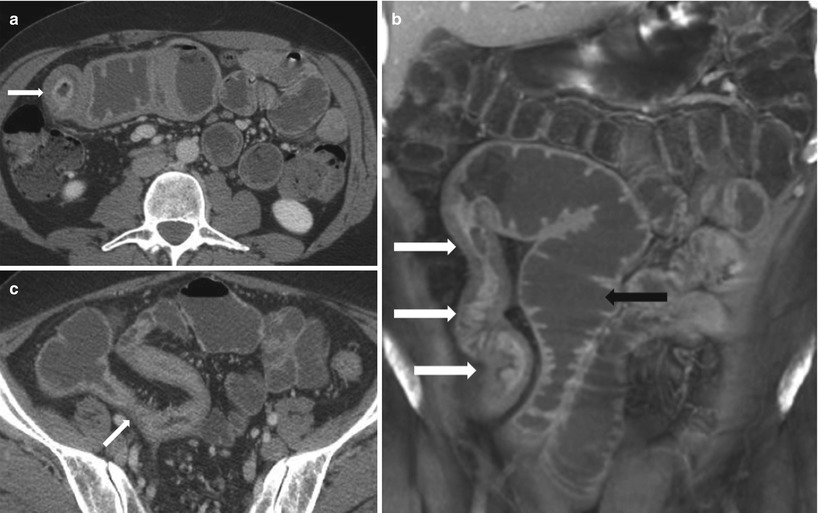
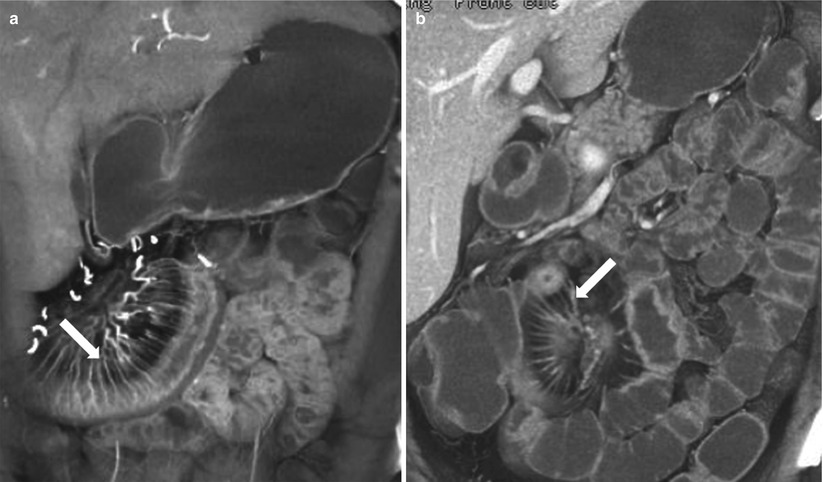

Fig. 10.12
Active Crohn’s disease. Axial contrast-enhanced CT images in three different patients (a–c) show mural stratification (closing parenthesis in c) with visualization of wall layers; mucosal hyperenhancement (white arrows), submucosal edema (transparent arrow) and enhancing serosa (arrowhead), surrounding mesenteric fat stranding, and prominent mesenteric vascularity

Fig. 10.13
Active Crohn’s disease with characteristic skip lesions. Axial contrast-enhanced CT images (a, b) in the same patient at two different levels show involvement of the small bowel at two segments (arrows) with active disease. Note, intervening segments of small bowel dilatation indicating partial small bowel obstruction

Fig. 10.14
Active and fibrotic strictures complicating Crohn’s disease. Axial (a) and coronal reconstructed (b) contrast enhanced CT images demonstrating long segment stricture of the terminal ileum with active disease indicated by bowel wall thickening with mural stratification causing luminal narrowing (white arrow). Note proximal small bowel dilatation indicating small bowel obstruction (black arrow). Axial contrast-enhanced CT (c) in a different patient demonstrates a homogenously enhancing wall suggestive of fibrotic stricture (arrow in c)

Fig. 10.15
Active Crohn’s disease with comb sign. Coronal reconstructed CT images in two different patients (a, b) show prominent vasa recta -comb sign (arrows), which is the most specific sign of active Crohn’s disease, that correlates with disease activity and elevated CRP levels
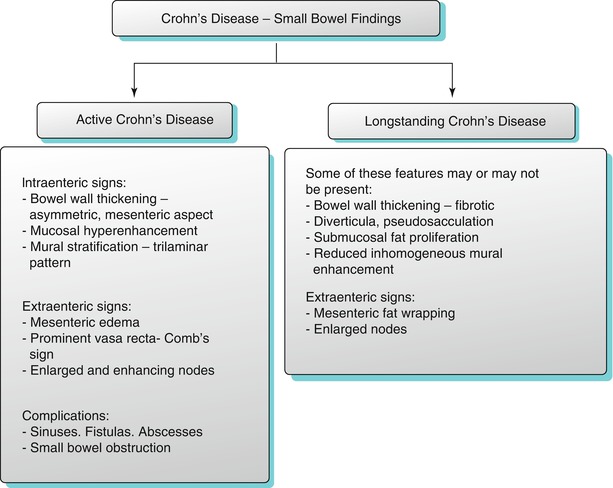
Magnetic resonance enterography is a noninvasive imaging technique increasingly being used in the assessment of complex or recurrent Crohn’s disease, allowing for the depiction of both intraenteric and extraenteric disease using multiplanar techniques without ionizing radiation. MR enteroclysis may hold a slight advantage over MR enterography in the detection of early disease. Due to the limited spatial resolution, the earliest changes of Crohn’s disease including mucosal nodularity, erythema, and superficial aphthous ulceration are not seen on cross-sectional imaging [28]. MR imaging findings in Crohn’s disease can predict the presence of active or long-standing disease (Algorithm 10.13). Findings are typically subdivided into intra- and extraenteric abnormalities.

Algorithm 10.13
Imaging features of active and long-standing Crohn’s disease
Intraenteric Disease on MR Enterography
Wall thickening in small bowel Crohn’s disease usually ranges between 5 and 10 mm [29] and is better assessed with a HASTE sequence due to the absence of a chemical shift artifact. Abnormal fold pattern is commonly recognized, with preferential involvement of the mesenteric border, which produces an asymmetric pattern of disease involvement. Three patterns have been described which include the following: picket fence, reduced or distorted folds due to ulceration, and cobblestone pattern. Ulceration is a common feature of the disease, with better depiction of moderate to deep ulcers on MR enterography, which show thin high-signal-intensity lines oriented longitudinally or transversely within the thickened bowel wall. Early or superficial ulceration may not be well demonstrated even with full luminal distention. Acute wall thickening represents disease activity. It may appear as a hyperintense stripe within the low T2 signal muscle on the fat-suppressed HASTE sequences and suggest the presence of mucosal or submucosal edema [30, 31] (Fig. 10.16). Small bowel strictures are well seen on MR enterography and appear as narrowed thick-walled segments of bowel with or without bowel obstruction. Fibrotic wall thickening appears as diffuse low to intermediate T2 signal within the muscle. Narrowed bowel segments may also be seen in adhesions, without wall thickening. Fatty infiltration of the bowel wall may occur in chronic IBD and can be differentiated from wall edema, with the use of fat saturation sequences or in-phase/opposed phase sequences which demonstrate chemical shift artifact (Fig. 10.17). Occasional fat within the bowel wall may occur in obese individuals [32]. Mural enhancement is a useful imaging feature to predict disease activity. The presence of layered [30, 33] or diffuse transmural enhancement usually suggests active disease, whereas the presence of reduced inhomogeneous enhancement suggests quiescent disease. The combination of bowel wall thickness greater than 4 mm and a ratio of greater than 1.3:1 between bowel wall enhancement and enhancement of normal bowel may be predictive of active disease [34]. Fibrotic strictures lead to apparent dilatation of the opposing bowel wall, especially along the antimesenteric border resulting in the formation of pseudosacculations, and dilatation of the bowel segment proximal to the fibrotic stricture.
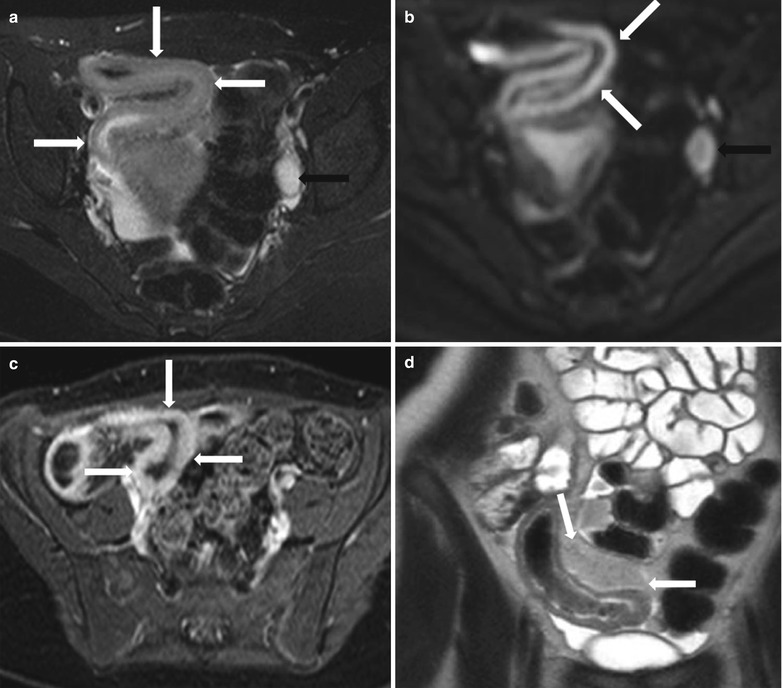
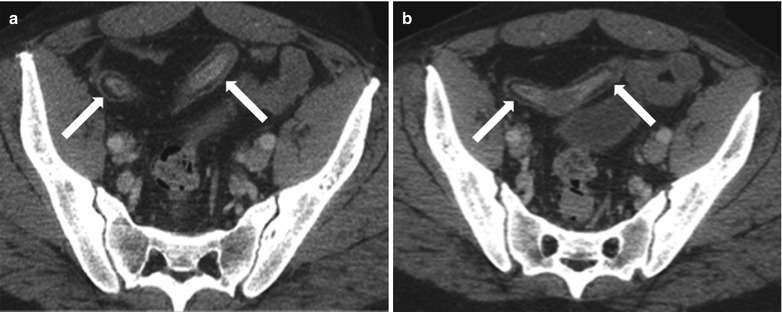

Fig. 10.16
MR enterography in active Crohn’s disease. Axial T2-weighted fat-suppressed (a), axial diffusion-weighted (b) and axial post-contrast fat-suppressed T1-weighted (c)images show mural thickening, with impeded diffusion and hyperenhancement in a long segment of distal ileum consistent with active inflammatory bowel disease (white arrows), with enlarged reactive lymph node along the left iliac chain (black arrows). Coronal T2-wighted image- HASTE (d) demonstrates marked fibrofatty proliferation (white arrows) adjacent to the distal ileum

Fig. 10.17
Submucosal fat deposition in the bowel wall associated with longstanding Crohn’s disease. Axial contrast enhanced CT images (a, b) show low attenuation of the submucosa secondary to submucosal fat deposition within the bowel wall (arrows)
Extraenteric Disease on CT Enterography
Extraenteric disease includes the presence of mesenteric edema, vascular engorgement, fat proliferation, mesenteric nodes, fistulae and sinuses, abscesses, and coexistent colonic abnormalities. Mesenteric edema is present in some cases with advanced active disease and is usually associated with bowel wall hyperemia and enhancement. Mesenteric vascular engorgement is called the comb sign as described above, and it appears as low-signal-intensity parallel lines on gradient echo images or high-signal-intensity enhancing parallel lines on post-contrast T1-w fat-suppressed images, oriented perpendicular to the longitudinal axis of the affected bowel wall. Fat wrapping is a pathognomonic sign of long-standing quiescent Crohn’s disease and represents asymmetric increased fat proliferation along the mesenteric border causing mass effect. Enhancing nodes typically lie along the vascular supply of an affected disease. Deep transmural ulcers may lead to the formation of enterocutaneous (Fig. 10.18), entero-enteric (Fig. 10.19), or enterovesical (Fig. 10.20) fistulae, which appear as enhancing high-signal-intensity tracts. These fistulous or sinus tracts may be associated with abscesses (Fig. 10.21) or loculated peritoneal collections (Fig. 10.22). Lastly, coexistent colonic abnormalities may be seen, which tend to mimic the small bowel findings. Additional findings include sacroiliitis or renal calculi.
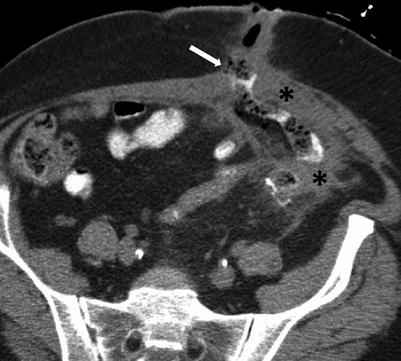
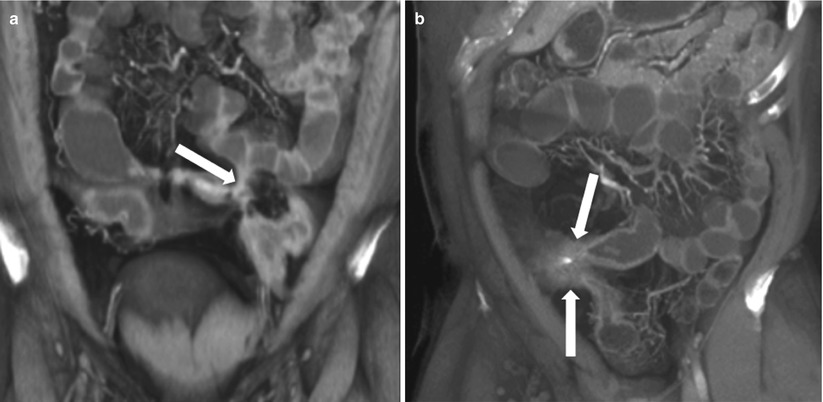
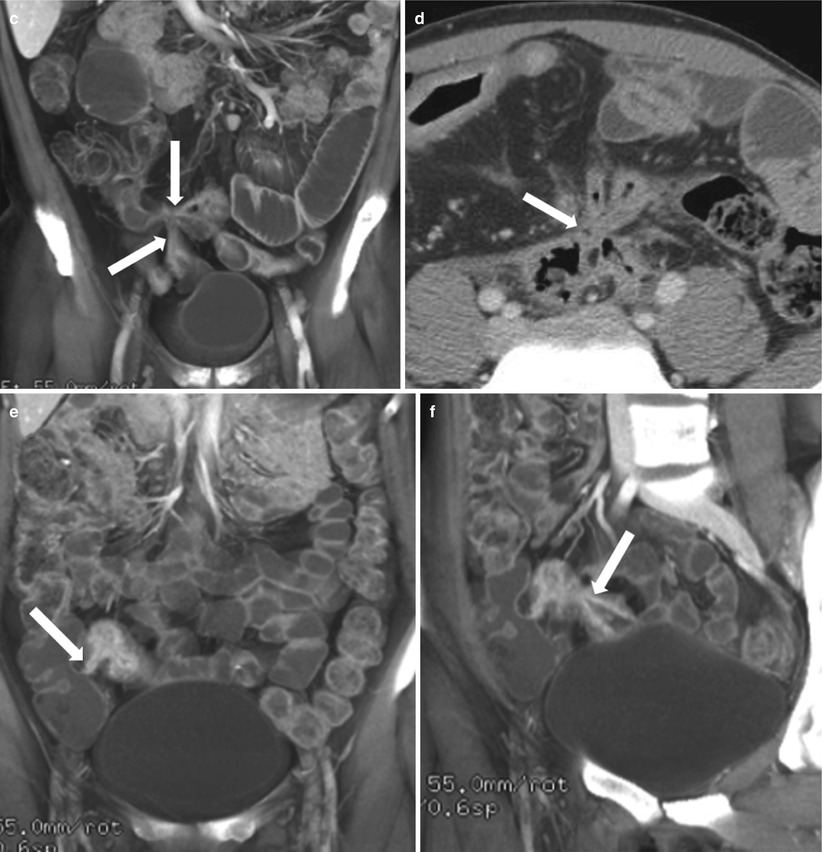
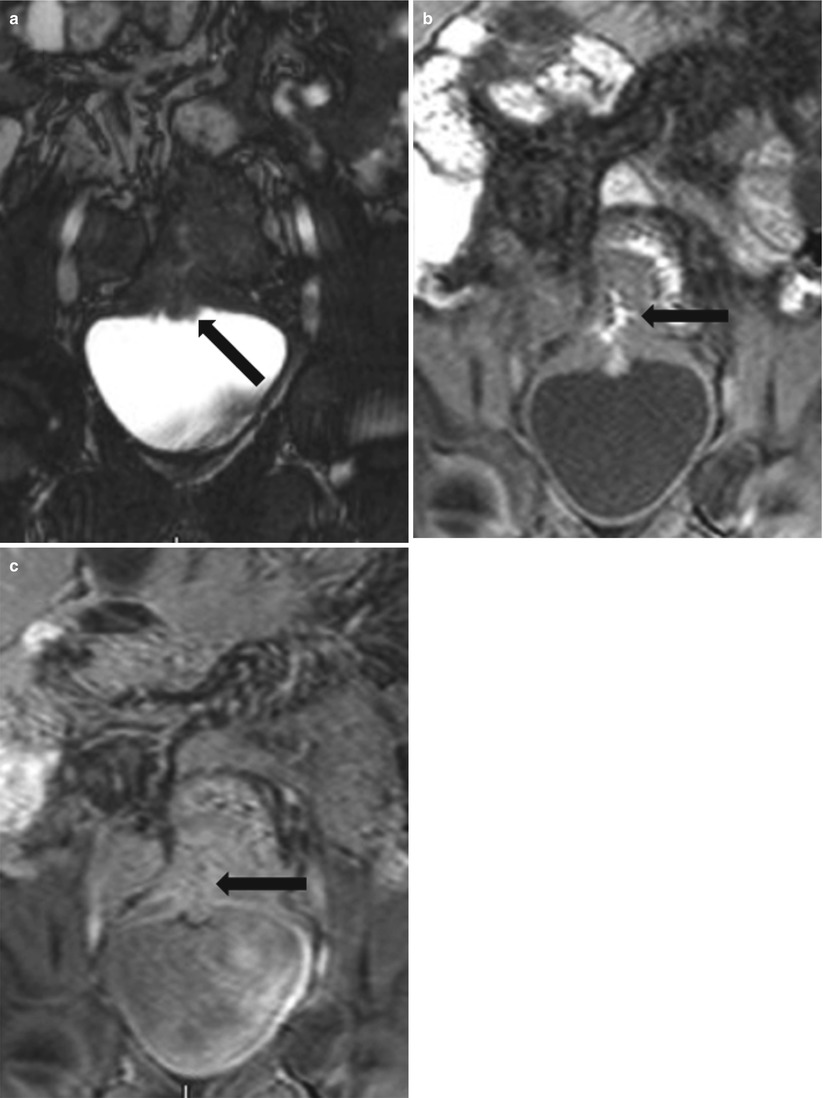
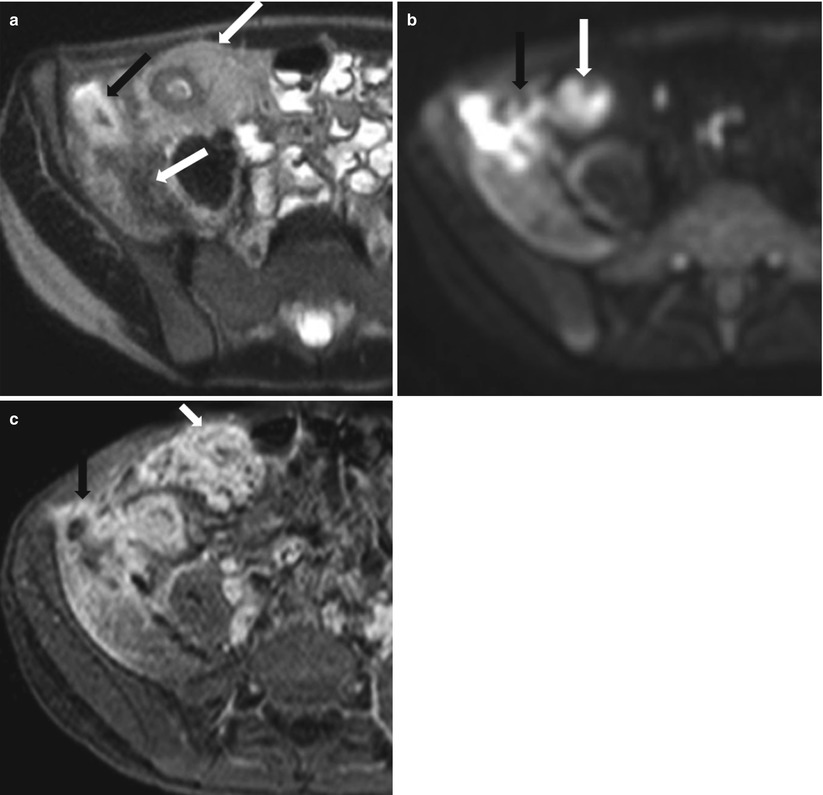
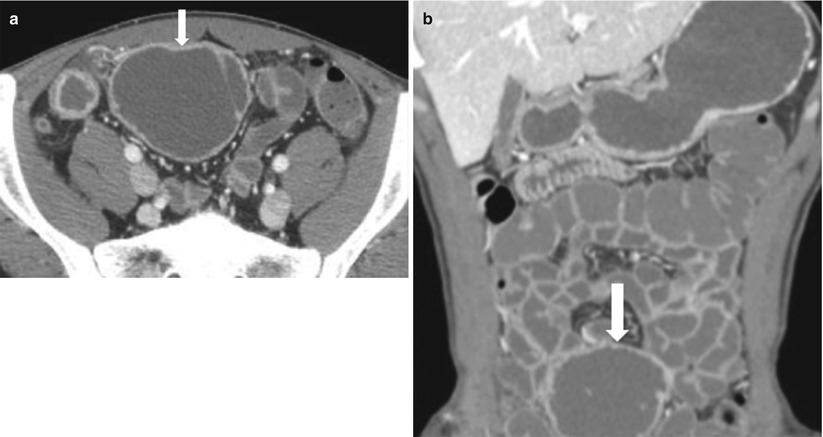

Fig. 10.18
Enterocutaneous fistula in Crohn’s disease. Axial contrast-enhanced CT of the abdomen shows extravasation of oral contrast from an ileal loop through a hyperattenuating fistulous tract (white arrow) communicating with the skin. Note perienteric inflammatory changes soft tissue thickening (black asterisks)


Fig. 10.19
Entero-enteric fistulae in Crohn’s disease. Ileo-ileal and ileocolic fistulas in active Crohn’s disease: Coronal (a–c and e), axial (d) and Sagittal (f) contrast enhanced CTE images show various examples of ileo-ileal and ileocolic fistulae in patients with Crohn’s disease appearing as hyperenhancing tracts connecting adjacent bowel loops (arrows)

Fig. 10.20
MR in active Crohn’s disease with an ileo-vesical fistula. Gradient echo coronal (a) image shows nodular thickening along the urinary bladder dome (black arrow). Non-contrast 3D T1W gradient echo (b) image shows oral enteric contrast containing fistulous tract (black arrow) between the thick inflamed ileum and bladder dome. Post-contrast 3D T1W gradient echo (c) image shows an enhancing fistulous tract (black arrow)

Fig. 10.21
MRI in active Crohn’s disease with an ileal fistula and iliacus abscess. Axial T2-weighted HASTE (a), diffusion-weighted (b) and post-contrast 3D T1-weighted fat-suppressed (c) images show bowel wall thickening and enhancement of the distal ileum with surrounding fibrofatty proliferation compatible with active Crohn’s disease (white arrows). Note the ileal fistulous tract leading to iliacus muscle abscess, with the characteristic impeded diffusion (black arrows)

Fig. 10.22
CT in active Crohn’s disease with a peri-ileal collection. Fluid collection in active Crohn’s disease. Axial (a) and coronal (b) CTE demonstrating a fluid collection (arrows). Note that though the contents of the collection demonstrate attenuation similar to enteric contrast, the lack of communication with bowel and caliber discrepancy confirms its extraluminal location
Note that though the contents of the collection demonstrate attenuation similar to enteric contrast, the lack of communication with bowel and caliber discrepancy confirms its extraluminal location.
Ulcerative Colitis
Ulcerative colitis predominantly involves the large bowel leading to proctocolitis (Fig. 10.23); however, the terminal ileum may be involved, which is termed as backwash ileitis. The pattern of involvement is typically continuous without evidence of skip lesions (Algorithm 10.14). Mural thickening is the specific feature. Pneumatosis and perforation may occur. In addition, rectal strictures, presacral fat accumulation, and rectal carcinoma [35] may also be identified. Colonoscopy is the primary diagnostic method for investigating ulcerative colitis [36]. Capsule endoscopy is useful to evaluate mucosal abnormalities of the small bowel, including pseudopolyp formations. CT enterography is primarily used to exclude small bowel involvement. MR enterography may provide information in cases in which endoscopy is inadequate and may depict mucosal abnormalities, wall thickening, mural stratification, and strictures.
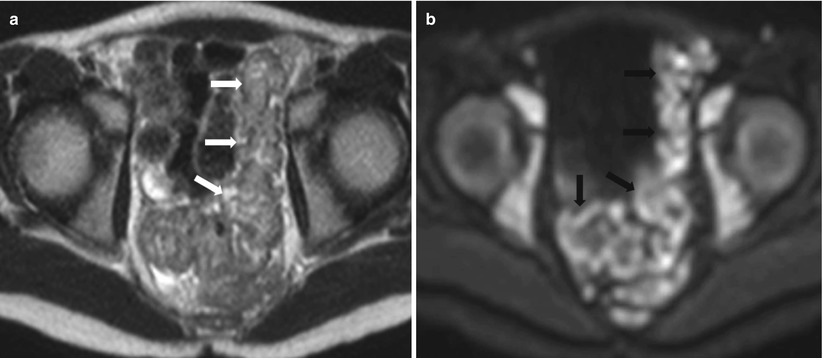
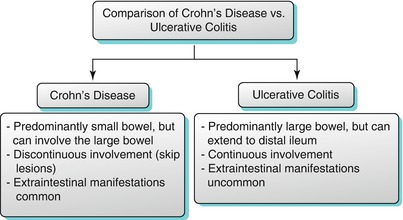

Fig. 10.23
Active proctocolitis in ulcerative colitis. Axial T2-weighted HASTE (a) and diffusion-weighted (b) images of the pelvis show thick and edematous wall of the rectosigmoid showing intermediate T2 signal intensity (white arrows), with impeded diffusion (black arrows) on DWI

Algorithm 10.14
Comparison of the salient imaging features of Crohn’s disease with those of ulcerative colitis
Celiac Disease
Celiac disease is an autoimmune disorder, which represents chronic intolerance to gluten. Celiac disease primarily affects the small bowel mucosa, resulting in progressive villus inflammation and destruction with induction of crypt hyperplasia. The definitive diagnosis of celiac disease requires both duodenal biopsies and a favorable response to gluten-free diet [37, 38]. CTE is useful in demonstrating features indicative or even specific of the diagnosis in patients with celiac disease [39–43]. The key imaging features of celiac disease are listed in Algorithm 10.15.
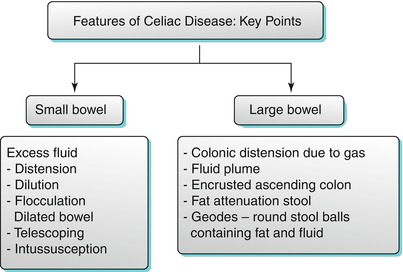

Algorithm 10.15
Features of celiac disease – key points
Common CT enterographic findings include characteristic features of malabsorption pattern involving the small and large bowel – duodenitis, dilution, small bowel dilatation, slow transit, flocculation, small bowel intussusception, villous atrophy, jejunization of the ileum (Fig. 10.24), colonic malabsorption pattern, hyposplenism, adenopathy, and celiac-associated T-cell lymphoma. The various causes of malabsorption are discusses in Algorithm 10.16.
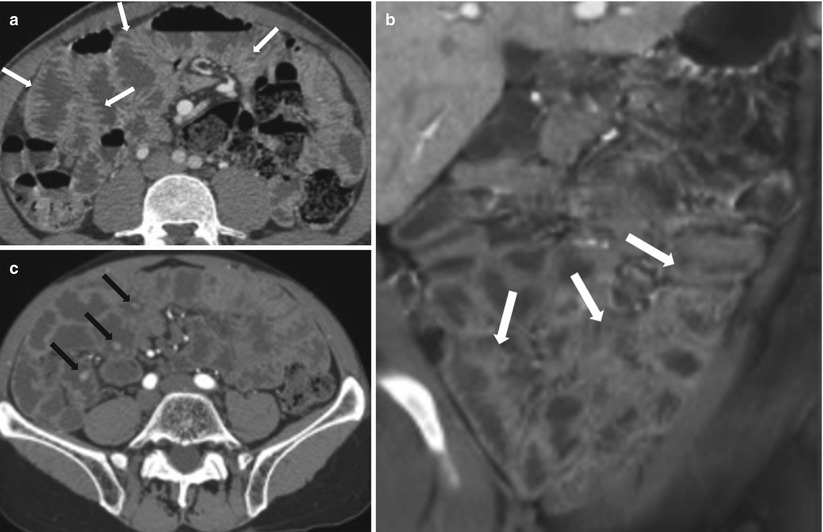
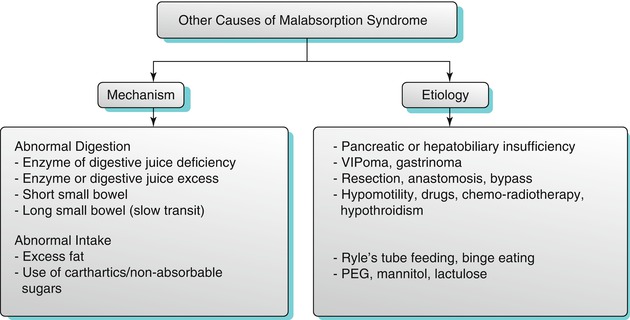

Fig. 10.24
Celiac disease. Axial and coronal CTE images (a, b) demonstrate jejunization of the mucosal pattern of the ileum (white arrows). Axial post-contrast CT (c) of another patient shows multiple polypoidal lesions involving the small bowel (black arrows)

Algorithm 10.16
Other causes of malabsorption syndrome
Reversal of jejunoileal fold pattern is typical of celiac disease [39, 40, 44] and is characterized by paucity or loss of jejunal folds and greater number of ileal folds (ileal jejunization). Lymphadenopathy is most marked in the upper small bowel mesentery and is due to follicular hyperplasia caused by proliferation of reactive B and T lymphocytes. Cavitating and low-attenuation nodes with fat-fluid levels have been reported in the advanced stages of the disease [45, 46]. Nodal cavitation is probably due to mesenteric lymphoid depletion and antigenic material crossing abnormal intestinal mucosa [47, 48]. Other features include hypervascular small bowel mesentery, edematous mesentery, and intramural fat in the small and large bowel.
Graft-Versus-Host Disease (GVHD)
Another diagnostic consideration in patients with diffuse small bowel thickening is graft-versus-host disease. This occurs when the immunocompetent graft reacts to the immune-incompetent host. GVHD of the bowel is frequently seen after bone marrow transplantation. The acute form primarily affects the small bowel. The CT appearance is nonspecific with diffuse mucosal enhancement and bowel wall thickening (Fig. 10.25). Small bowel involvement is more frequent than large bowel involvement. Diffuse involvement of small bowel is more often seen than segmental involvement. Ascites is frequently present. Long-term sequelae include strictures [49].
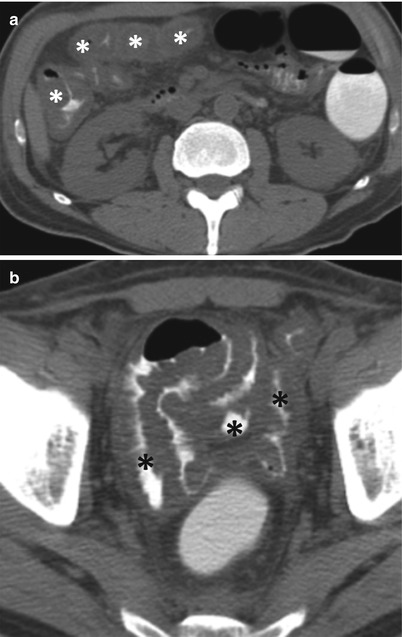

Fig. 10.25
Graft-versus-host disease. Axial non-enhanced CT images (a, b) show diffuse mural thickening of dilated large (white asterisks) and small bowel (black asterisks) loops, with diffuse wall thickening, with minimal ascites
Radiation Enteritis
Radiation enteritis occurs when radiation therapy exceeds 45 Gy with resultant obliterative vasculitis in the intestinal wall and mesentery. The ileum is predominantly affected with changes also in the colorectal bowel. Complications include fistulae and bowel obstruction. In early disease, affected bowel segments show homogeneous wall thickening with progressive luminal narrowing and irregular outer wall contours (Fig. 10.26). The circumferentially thickened hypoattenuating submucosal layer produces a target pattern in the bowel wall. Perirectal and mesenteric fibrotic strands with thickened fascial planes and peritoneal adhesions are seen. In late radiation enteritis, fibrosis leads to minimally enhancing narrowed bowel with varying degrees of proximal dilatation. Perirectal fat proliferation may be present. The affected bowel loops are those involved in the radiation port [50].
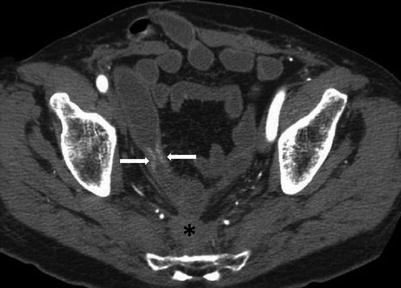

Fig. 10.26
Radiation enteritis. Axial CTE image shows a short segment stricture of the small bowel secondary to radiation enteritis (white arrows). Note diffuse hypoattenuating presacral soft tissue, with thickening of the retro-rectal fat planes (black asterisk)
Eosinophilic Enteritis
Eosinophilic enteritis is an uncommon inflammatory disease characterized by focal or diffuse eosinophilic infiltration of the GI tract, more commonly the stomach and esophagus. An allergic disorder and peripheral eosinophilia are present in most patients. The most common CT abnormality is bowel stenosis and fold thickening. Most patients respond well to steroid therapy. The CT appearance can mimic the inflammatory appearance of Crohn’s disease including bowel wall thickening, luminal narrowing without obstruction, mesenteric lymphadenopathy with peripheral rim-like enhancement and marked necrosis, and possible ascites. CT findings are more characteristic of an inflammatory disease rather than a tumor, and these findings are helpful for assessing the extent and location of the disease and guiding biopsy [51].
Scleroderma
Gastrointestinal manifestations of scleroderma can occur in up to 90 % of patients with scleroderma with the commonest site of GI involvement being the esophagus. Smooth muscle atrophy and fibrosis is thought to be the chief underlying mechanism which leads to luminal dilatation, reduced motility, and reduced sphincter tone. Small bowel is affected in more than 60 % of scleroderma patients, the duodenum most frequently involved. Patients may be asymptomatic or may present with bloating or malabsorption due to bacterial overgrowth. The radiographic features include luminal dilatation (can be massive), hidebound bowel sign (crowding of valvulae conniventes – thought to be pathognomonic of scleroderma), accordion sign (well seen evenly spaced mucosal folds in duodenum), and sacculations (focal dilatations or pseudo-diverticula along the antimesenteric border). Differential diagnoses include sprue and small bowel obstruction.
Amyloidosis
Amyloidosis is defined as the extracellular deposition of protein fibers with a β-sheet fibrillary structure. The gastrointestinal tract is involved in 98 % of cases of systemic amyloidosis, with colonic involvement being most common. Small bowel involvement results in abdominal pain, malabsorption, hemorrhage, and perforation [52].
CT findings are nonspecific and tend to overlap with inflammatory bowel disease. Findings include focal or diffuse bowel wall thickening, thickened mucosal pattern, jejunization of ileum, intramural hemorrhage, bowel dilatation and obstruction, and rarely perforation. Other features include mesenteric infiltration and adenopathy. Occasionally nodular or diffuse omental [53] and peritoneal infiltration [53, 54] is seen, with the nodular deposits representing enlarged nodes and the diffuse form representing infiltration of the omental or retroperitoneal fat. Secondary calcification of the omental deposits is characteristic of amyloidosis [55]. Extraintestinal manifestations include splenomegaly and diffuse hepatic and gallbladder wall infiltration.
Brunner’s Gland Hyperplasia and Hamartoma
Brunner’s glands are mucosal and submucosal glands that secrete mucus, pepsinogen, and urogastrone into the crypts of Lieberkuhn. Majority of these glands are seen proximal to the ampulla of Vater, with gradual reduction in the distal duodenum and few located in the prepyloric region of the stomach. Pathologic changes involving Brunner’s gland range from hyperplasia, hamartoma, and adenoma, with the terms being used interchangeably. Lesions less than 5 mm in size are termed hyperplasia and those greater than 5 mm in size are termed hamartoma [56]. Brunner’s gland hyperplasia is an incidental finding on 2 % of gastroscopies [57]. Typically, these are considered benign entities; however, associated dysplasia or adenocarcinomas have been reported.
On US, Brunner’s glands reveal heterogeneous hyperechogenicity, with hamartomas appearing as heterogeneous lesions having an indistinct margin [58]. Endoscopic ultrasound is useful in detecting submucosal lesions and when biopsies need to be performed. On NECT, these lesions appear iso-attenuating relative to the pancreas. Following administration of contrast, the lesions appear hypoattenuating to the pancreas, surrounded by a peripheral enhancing rim, which represents the duodenal mucosa [59].
Nodular Lymphoid Hyperplasia
Nodular lymphoid hyperplasia is a rare disorder of the small bowel, often reported with immune deficiency disorders. NLH represents hyperplasia of the lymphoid follicles with large germinal centers located within the submucosa or lamina propria and may present either as a localized segmental or diffuse form. The disorder is characterized by the presence of numerous mucosal nodules, usually measuring less than 0.5 cm in diameter. NLH has a potential risk for developing both intestinal and extraintestinal lymphoma [60]. NLH may be associated with Giardia lamblia infection [61], common variable immunodeficiency [62], Familial Adenomatous Polyposis (FAP) [63], Gardner’s syndrome [63], and HIV [64]. CT imaging may show mural thickening, polypoid masses, nodular fold thickening, separated bowel loops, and enlarged retroperitoneal nodes [65].
Infections
Tuberculosis
Tuberculosis may involve any segment of the gastrointestinal tract, with preponderance for the terminal ileum, ileocecal valve, and cecum [66], primarily due to the abundance of lymphoid tissue in this region associated with relative stasis. Intestinal tuberculosis may produce small bowel obstruction [67]. In the early stages, bowel wall thickening with slight luminal narrowing and few regional adenopathy may only be identified. In later stages, the transmural wall thickening produces severe concentric luminal narrowing with or without proximal bowel dilatation, distortion of the ileocecal valve, and enlarged necrotic and non-necrotic locoregional nodal masses (Fig. 10.27). Associated findings include multifocal involvement of the small or large bowel, mesenteric haziness, omental and peritoneal nodules, and coexistent extra-abdominal lesions. In chronic cases or following antituberculous therapy, healing may be associated with the formation of fibrotic strictures and marked distortion of the cecal pole.
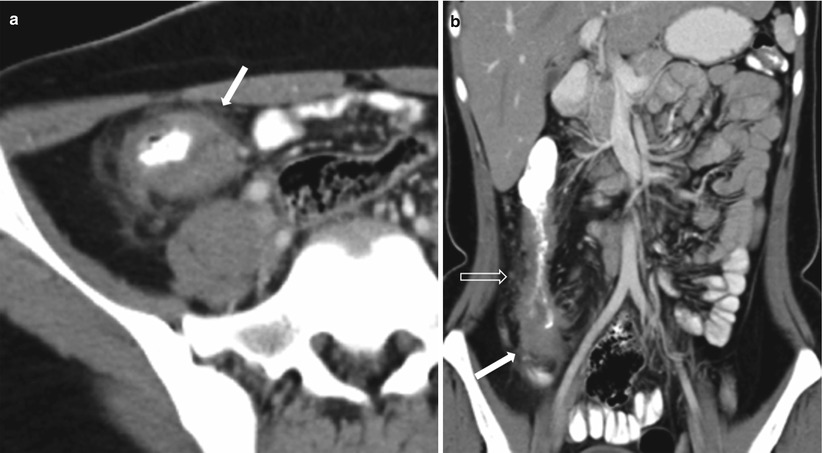

Fig. 10.27
Ileocecal tuberculosis.Axial (a) and coronal (b) contrast-enhanced CT images obtained in the venous phase show terminal ileal and cecal thickening (white arrows), with perienteric fat stranding and enlarged locoregional nodes (transparent arrow)
HIV and Small Bowel
Gastrointestinal disorders are among the most frequent presenting complaints in patients with HIV. In HIV patients, multifocal abnormalities are a common occurrence, including the presence of multiple and widely disseminated opportunistic infections, multiple foci of Kaposi’s sarcoma, coexistence of opportunistic infections and tumor, or even multiple coexisting tumors such as lymphoma or Kaposi’s sarcoma. Various forms of small bowel involvement in HIV correlates with CD4 levels (algorithm 10.17).
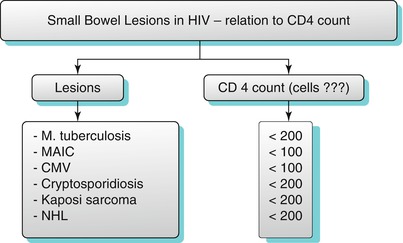

Algorithm 10.17
Small bowel lesions in HIV-relation to CD4 count
Common opportunistic infections involving the small bowel include CMV, cryptosporidiosis, MAIC, tuberculosis, and bacillary angiomatosis (Algorithm 10.17).
CMV
CMV typically affects the small bowel and colon, the clinicopathological and radiographic manifestations of which simulates inflammatory bowel disease or ischemic colitis. Contrast-enhanced CT studies in patients with mild to moderate CMV enteritis may reveal intense mucosal enhancement, target pattern of bowel wall thickening [68], prominent submucosal nodular projections due to lymphoid nodular hyperplasia, submucosal hemorrhage, perienteric fat stranding and fluid, and perforation. Usually, the cecum and ascending colon are also involved due to coexistent colitis. Lymphadenopathy is an uncommon finding in CMV enteritis.
Cryptosporidiosis
MAIC
Immunocompromised patients have an increased incidence of infections with atypical mycobacteria, particularly MAIC and clinically present with abdominal pain, diarrhea, and weight loss. The most common CT findings are small bowel wall thickening, enlarged retroperitoneal and mesenteric lymph nodes, and hepatosplenomegaly [71]. Retroperitoneal and mesenteric low-attenuation lymphadenopathy is usually seen [72].
Tuberculosis
Tuberculosis of the small bowel has been described in detail in the previous section.
Small Bowel Neoplasms
Small bowel tumors are rare, constituting approximately 3–6 % of all gastrointestinal neoplasms. The detection of small bowel neoplasms is difficult, as the presenting symptoms are nonspecific, which include pain, hemorrhage, or anemia, and the clinical suspicion is very low. Endoscopy and conventional enteroclysis are routinely used to evaluate for mucosal abnormalities but are limited in evaluating the mural and extramural extent of lesions. Techniques including CT and MRI combine the benefits of cross-sectional imaging and enteroclysis to evaluate the small bowel. The small bowel is afflicted by a wide spectrum of primary neoplasms, which are enumerated in Algorithm 10.18. Some features can help in the diagnosis of the most commonly encountered small bowel masses (Table 10.1).
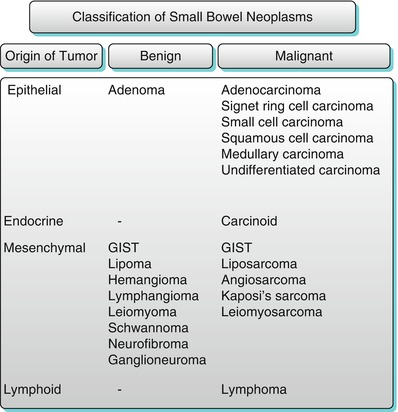

Algorithm 10.18
Classification of small bowel neoplasms
Table 10.1
Helpful features for diagnosis of the most commonly encountered small bowel masses
Leiomyoma: Typically well-circumscribed homogenously enhancing mass |
Lipoma: Fat containing well-circumscribed mass |
GIST: Typicall exophytic |
Lymphoma: Typically polypoid. No mass effect. Often aneurysmal dilatation |
Adenocarcinoma: Can have various patterns; infiltrative, polypoidal, irregular/heterogeneous; could be associated with obstruction
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|