Chapter 12 The Trachea
RADIOGRAPHIC EVALUATION
Plain Films
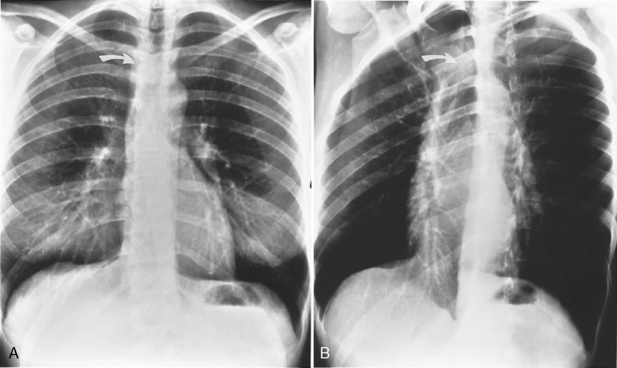
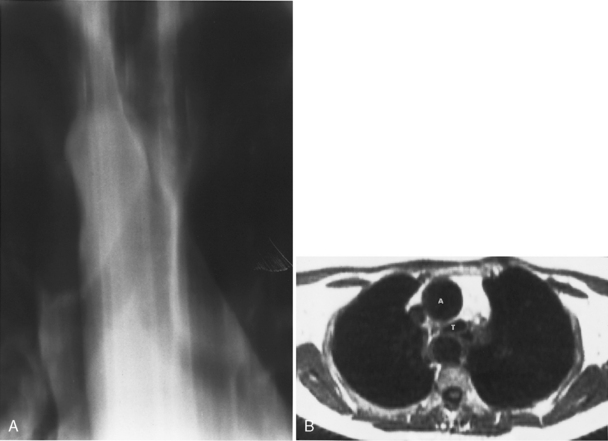
Several radiographic studies are used to evaluate the trachea and main bronchi (Table 12-1). The plain chest radiograph in the posteroanterior and lateral projections is the most frequently used screening study. A high-kilovoltage (140-kVp) technique is preferred because it reduces the visibility of the bony thorax and improves imaging of the various mediastinal interfaces. However, it is easy to miss a tracheal or main bronchial lesion on standard posteroanterior and lateral chest radiographs because there is considerable overlap of the trachea with the mediastinum and bony thorax. Bilateral, oblique chest radiographs improve visibility of the trachea and main bronchi by rotating the spine so that it is not superimposed on the central airways (Fig. 12-1). In most cases, additional imaging is usually indicated.
Table 12-1 Approach to Diagnostic Imaging of the Trachea
| Study | Clinical Indications |
|---|---|
| Chest radiographs (posteroanterior, lateral, oblique projections) | Screening study |
| Conventional tomography (anteroposterior, lateral, 55-degree posterior oblique projections) | Postintubation tracheal stenosis Preoperative assessment of length of lesion Postoperative assessment of bronchial anastomosis |
| Computed tomography | Tracheobronchial tumor location and extent Density determination Vascularity of tumor Airway diameter Wall thickness Tracheomalacia Compression of airway by mediastinal mass or vessel Tracheobronchial rupture Tracheobronchial dehiscence |
| Magnetic resonance imaging | Multiplanar imaging Mediastinal invasion by airway, neoplasm Airway obstruction by vascular rings |
| Fluoroscopy | Tracheomalacia Air trapping due to bronchial obstruction |
Computed Tomography
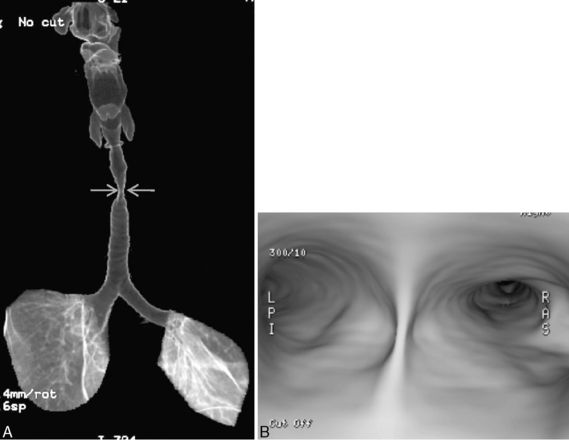
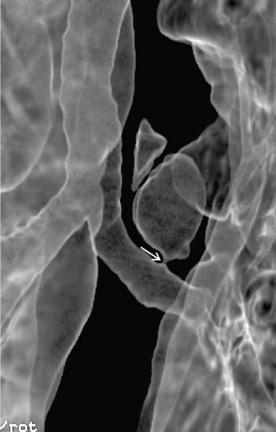
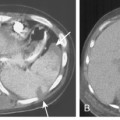
Multidetector CT (MDCT) has significantly improved the way the trachea is imaged. MDCT increases the capacity to register data and significantly shortens the scan time. The entire trachea and major bronchi can be imaged in a single breath hold of a few seconds. The isometric data set obtained with MDCT has equal resolution in all the imaging planes, and artifacts seen with multiplanar reformats in conventional CT can be avoided. High-quality, multiplanar images are possible if thin sections (1 to 2.5 mm) and an overlapping image reconstruction algorithm are used (Fig. 12-2). The image reconstruction thickness can be selected prospectively or retrospectively after raw data set is acquired. The same data can be transferred to a dedicated workstation to obtain three-dimensional images. The two commonly used three-dimensional techniques are external volume rendering of the airways (Fig. 12-3A) and internal rendering or virtual bronchoscopic views (see Fig. 12-3B). These imaging techniques supplement but do not replace conventional axial images. There are several advantages of using these techniques. The craniocaudal extent and shape of the lesion is better displayed, complex and subtle lesions can be better demonstrated (Fig. 12-4), and simulated bronchoscopic views can be used as a guide in planning interventional procedures.
Magnetic Resonance Imaging
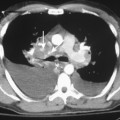
The role of magnetic resonance imaging (MRI) in the evaluation of the trachea and bronchi is limited. The trachea, main bronchi, and lobar bronchi are well demonstrated on spin echo images, but the spatial resolution of MRI permits observation of only an occasional segmental bronchus. MRI offers the advantages of multiplanar imaging, high-contrast resolution without the use of intravenous contrast agents, and absence of ionizing radiation. MRI is particularly useful in patients with vascular rings or tracheal compression by vascular anomalies and other mediastinal masses (Fig. 12-5).
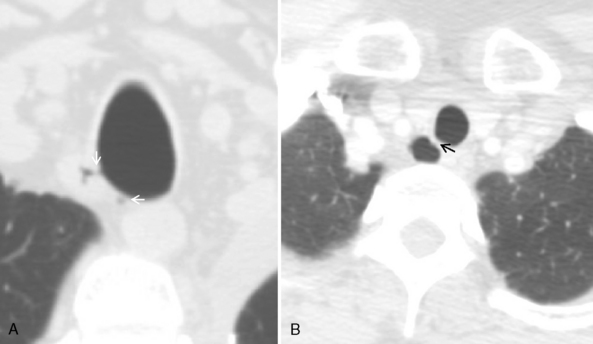
TRACHEAL DIVERTICULA
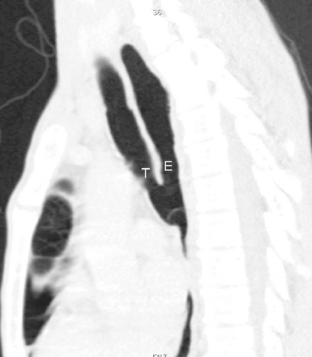
On standard radiographs, small diverticula are often missed. Thin-section CT with multiplanar and three-dimensional reconstructions establishes the relation to the trachea, and further imaging is usually not required (Fig. 12-6; see Fig. 12-4).
TRACHEAL STENOSIS
Diffuse tracheal narrowing is seen in several conditions, including chronic obstructive pulmonary disease (COPD); after tracheal trauma; as the result of viral, fungal, tuberculous, or bacterial infections; and in other diseases, such as sarcoidosis, Wegener’s granulomatosis, relapsing polychondritis, amyloidosis, and tracheobronchopathia osteochondroplastica. The narrowing may be idiopathic and may involve the larynx (Table 12-2).
Table 12-2 Diffuse Tracheal Stenosis
| Causative Conditions | Tracheobronchial Findings | Other Findings |
|---|---|---|
| Idiopathic | Smooth, tapered, irregular, lobulated, or eccentric morphology 2-4 cm long, usually subglottic | ±Laryngeal involvement |
| Postintubation cuff injury | Smooth narrowing with hourglass configuration | |
| Posttraumatic | Smooth narrowing with hourglass configuration | ±Upper rib and sternal fractures |
| Saber-sheath trachea | Smooth narrowing of intrathoracic trachea Coronal diameter ≤ one half of sagittal diameter | Hyperinflated lungs |
| Tracheopathia osteochondroplastica | Submucosal nodularity of anterolateral walls of trachea and main bronchi with ossification Membranous wall is spared | |
| Relapsing polychondritis | Diffusely thickened tracheobronchial walls with diffuse narrowing of trachea and main bronchi ±Calcification of wall ±Tracheobronchomalacia | Auricular and nasal chondritis, arthritis |
| Wegener’s granulomatosis | Focal or diffuse tracheobronchial wall thickening and narrowing ±Enlarged calcified cartilages | Renal involvement |
| Amyloidosis | Diffuse tracheobronchial wall-thickening and narrowing Focal nodular masses ±Calcification Contrast enhancement of masses on CT or MRI Slowly progressive | ±Lymphadenopathy, may calcify |
| Sarcoidosis | Smooth, irregular, or nodular stenosis Tracheobronchial wall thickening Bronchial compression by lymph node | ±Lymphadenopathy, ±calcification ±Reticular/nodular interstitial lung disease |
| Infectious | ||
| Tracheobronchial papillomatosis | Diffuse nodules or masses in trachea and bronchi | Laryngeal involvement ±Multiple pulmonary nodules, may cavitate Complicated by squamous cell carcinoma |
| Rhinosclerosis | Nodular masses or diffuse, symmetric narrowing of trachea and bronchi Slowly progressive | |
| Tuberculosis | ||
| Hyperplastic stage | Irregular tracheobronchial wall thickening and narrowing | Hilar/mediastinal lymphadenopathy Parenchymal cavitation and consolidation |
| Fibrostenotic stage | Smooth tracheobronchial narrowing | Atelectasis and scarring Bronchiectasis Calcified lymph nodes |
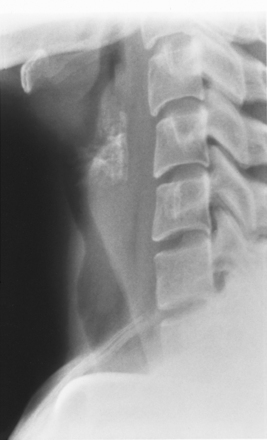
Idiopathic Laryngotracheal Stenosis
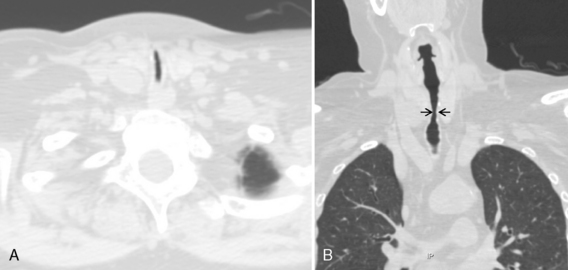
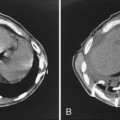
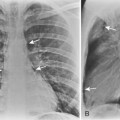
The radiologic appearance of idiopathic laryngotracheal stenosis varies, including lesions that may be smooth and tapered (Fig. 12-7) or irregular, lobulated, and eccentric (Fig. 12-8). The stenosis is 2 to 4 cm long, with severe compromise of the lumen measuring no more than 5 mm at the narrowest point.
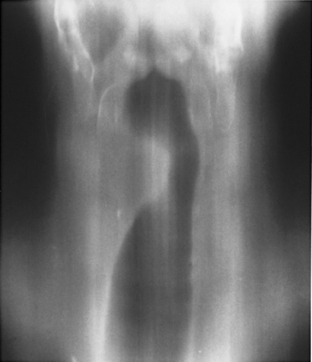
Postintubation Injuries
Most long-term complications of intubation are related to cuff injury. Cuffed endotracheal tubes became commonplace only after the introduction of intermittent positive-pressure breathing for the treatment of respiratory failure during the poliomyelitis epidemic of 1952. As patients survived for longer periods with respiratory assistance, new long-term complications arose, including tracheal stenosis, tracheoesophageal fistula, and tracheoinnominate artery fistula (Box 12-1).
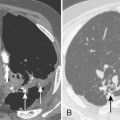
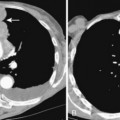
A stenosis may occur at the level of the tracheostomy stoma or rarely where the tip of the tube impinges on the tracheal mucosa. However, pressure necrosis at the cuff site is responsible for most long-term postintubation complications. If the cuff pressure exceeds capillary pressure, blood supply to the mucosa is compromised, leading to ischemic necrosis. Initially, the mucosa becomes inflamed, followed by ulceration in the mucosa overlying the cartilaginous rings. As the ulcers enlarge, there is increasing exposure of the cartilage, which becomes colonized with bacteria. With further pressure on the wall, necrosis develops, and softening and dissolution of the cartilage lead to tracheomalacia or to scarring and stenosis formation. Radiographically, the stenosis has a smooth, gradual narrowing with an hourglass configuration (Fig. 12-9). Typically, symptoms of stenosis develop in 2 to 6 weeks after extubation and, in some patients, months later. Most stenoses are appropriately treated with resection of the damaged segment and end-to-end anastomosis. In the past 20 years, most tracheostomy and endotracheal tubes have been designed with large-volume, low-pressure cuffs, which has dramatically reduced the incidence of tracheal stricture. Postintubation tracheal strictures continue to occur but at a much reduced rate.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree