Figure 8.1.1
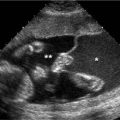
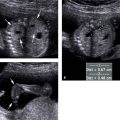
Figure 8.1.1
Cystic adenomatoid malformation type 1. A: Axial image of chest demonstrating large echogenic mass (arrows) with a large cyst (*). The mass fills the right hemithorax, displacing the heart (arrowhead) to the left. B: Sagittal image of right chest showing the large mass (arrows) containing the cyst (*).
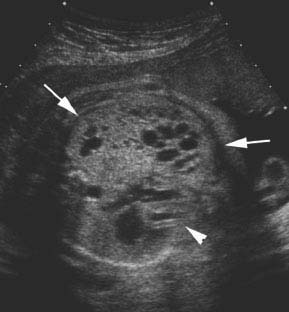
 Figure 8.1.2
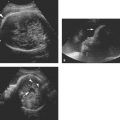
Figure 8.1.2
Cystic adenomatoid malformation type 2. Axial image of chest demonstrating large echogenic mass (arrows) containing small cysts, displacing the heart (arrowhead) to the right.
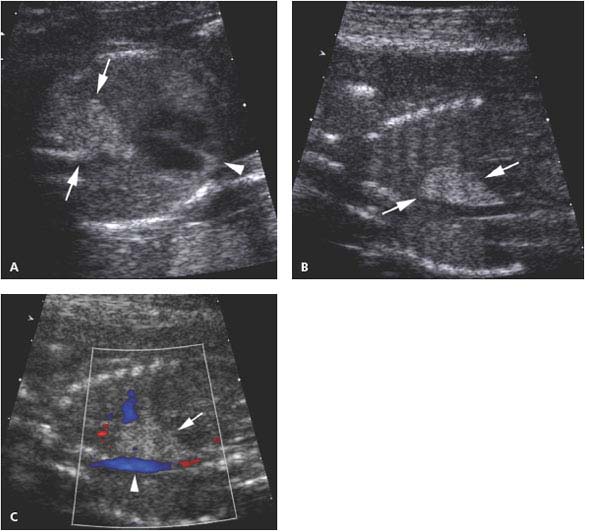
Figure 8.1.3
Cystic adenomatoid malformation type 3. A: Axial image of chest showing echogenic mass (arrows) in posterior right chest. The heart (arrowhead) is not displaced. B: Sagittal image of fetal right chest showing that the echogenic mass (arrows) replaces the right lower lobe of the lung. C: Sagittal image of right chest with color Doppler showing that no vessel travels from the thoracic aorta (arrowhead) to the mass (arrow).
Type 3 lesions have no visible cysts, because the cysts are too small to be seen with ultrasound. When the entire lung is involved, the appearance of a type 3 cystic adenomatoid malformation is the same as that of bronchial atresia.
Color Doppler can be used to determine that the blood supply to the mass arises from the pulmonary artery (Figure 8.1.4). Sometimes, the cystic lung mass will decrease in size during gestation (Figure 8.1.5). If the cystic adenomatoid malformation is large, it may cause mediastinal shift or inversion of the diaphragm, and the fetus may have ascites, pleural effusions, or full-blown hydrops (Figure 8.1.6).
A sequestration appears on ultrasound as a homogeneously echogenic mass located either within the thorax (Figure 8.1.7) or just below the diaphragm (Figure 8.1.8). Color Doppler is used to identify the feeding artery from the aorta (Figure 8.1.9), confirming the systemic blood supply. This finding helps distinguish a sequestration from a type 3 (microcystic) cystic adenomatoid malformation of the lung, which receives its blood supply from the pulmonary artery.
Mixed pulmonary lesions, those with characteristics of both cystic adenomatoid malformation and sequestration, appear as cystic lung masses with systemic arterial blood supply (Figure 8.1.10).
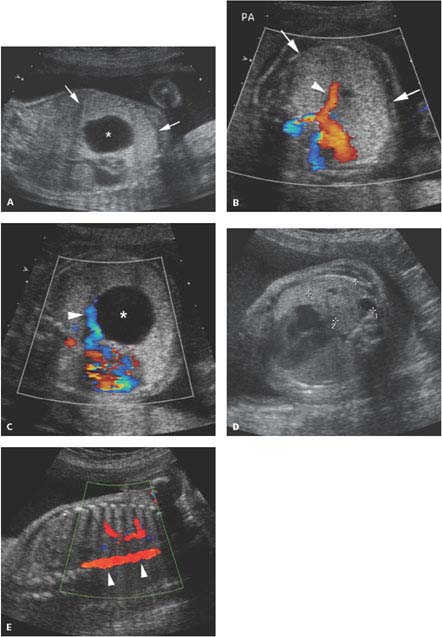
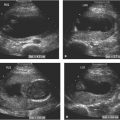
Figure 8.1.4
Color Doppler of pulmonary blood flow to cystic adenomatoid malformation. A: Sagittal image of fetal chest demonstrating large type 1 cystic adenomatoid malformation (arrows) with large cyst (*). B: Axial image of chest with color Doppler showing large branch of the pulmonary artery (arrowhead) carrying blood to the echogenic chest mass (arrows). C: Axial image of chest with color Doppler showing a pulmonary vein (arrowhead) draining the blood to the left atrium from the chest mass with its large cyst (*). D: Axial image of chest in another fetus showing a mass (calipers) with small cysts in posterior right chest. E: Color Doppler showing no vessel feeding this mass from the descending thoracic aorta (arrowheads), confirming its pulmonary arterial supply.
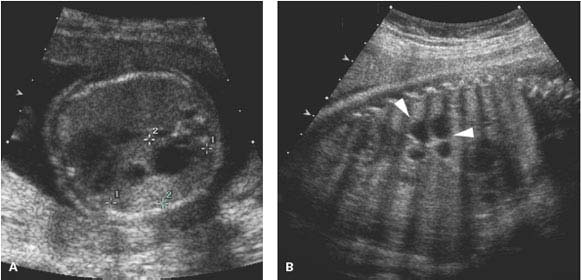
Figure 8.1.5
Cystic adenomatoid malformation partially regresses in utero. A: Axial image of chest of 21-week fetus demonstrating moderate size left chest mass (calipers) containing a few cysts. B: Sagittal image of chest in same fetus at 32 weeks gestation showing that the mass (arrowheads) is smaller in size.
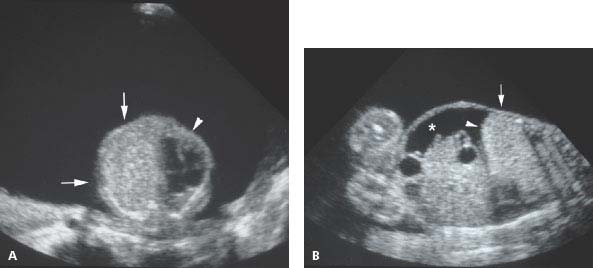
Figure 8.1.6
Large type 3 cystic adenomatoid malformation. A: Axial image of fetal chest showing large echogenic mass (arrow) in left thorax displacing the heart (arrowhead) to the right. Polyhydramnios is present. B: Coronal image of fetus demonstrating echogenic mass (arrow) filling right chest, causing inversion of the diaphragm (arrowhead) and accumulation of ascites (*).
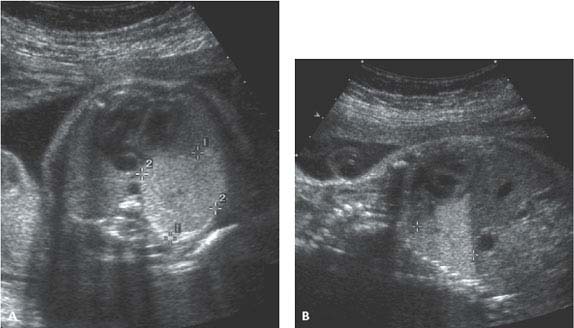
Figure 8.1.7
Bronchopulmonary sequestration. A: Axial and (B) sagittal images of fetal thorax showing wedge-shaped, homogeneously echogenic mass (calipers) in posterior inferior left chest, representing a left lower lobe sequestration.
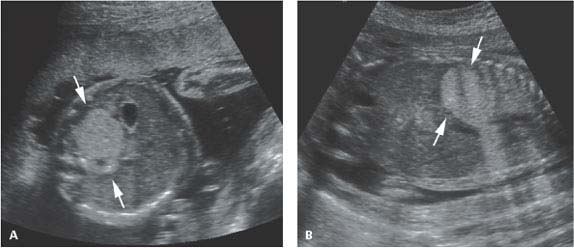
Figure 8.1.8
Subdiaphragmatic pulmonary sequestration. A: Axial image of upper abdomen showing homogeneously echogenic mass (arrows) posterior to stomach in left upper quadrant, representing a subdiaphragmatic sequestration. B: Coronal image showing the echogenic mass (arrows) in the left upper abdomen.
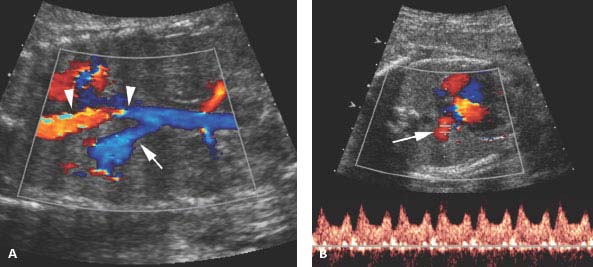
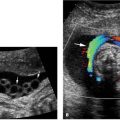
 Figure 8.1.9
Figure 8.1.9
Systemic arterial blood supply to bronchopulmonary sequestration. A: Sagittal image of chest with color Doppler showing a large artery (arrow) arising from the thoracic aorta (arrowheads) feeding the left lower lobe sequestration shown in Figure 8.1.7. B: Axial image with color and spectral Doppler showing that venous return from the sequestration is via a pulmonary vein (arrow) to the left atrium.
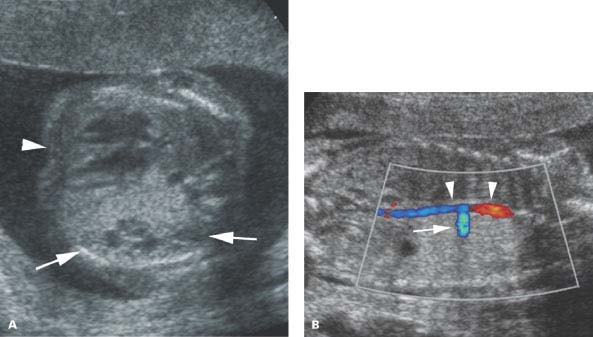
Figure 8.1.10
Pulmonary dysplasia, mixed type. A: Axial image of fetal thorax demonstrating left chest mass (arrows) with a few small cysts, displacing the heart (arrowhead) to the right. The mass has sonographic features of a cystic adenomatoid malformation. B: Coronal image of same fetus showing a large artery (arrow) arising from the descending thoracic aorta (arrowheads) feeding the left chest mass, a characteristic of pulmonary sequestration.
8.2. Tracheal and Bronchial Atresia
Description and Clinical Features
If the trachea is atretic, pulmonary secretions remain trapped behind the atresia in the lungs. This leads to distention of the lungs. The distended lungs compress the heart, preventing adequate venous return and causing hydrops. Tracheal atresia is likely to be fatal at birth. Even if the condition is diagnosed prenatally, establishing an airway at delivery may be impossible.
Atresia of a bronchus will lead to distention of one lung, causing mass effect, with shift of the heart and mediastinum to the contralateral side. The lung distal to the obstruction develops abnormally. The condition falls under the spectrum of pulmonary dysplasias, as it is similar or identical to cystic adenomatoid malformation type 3 involving the entire lung.
Sonography
With tracheal atresia, both lungs are large and homogeneously echogenic (Figure 8.2.1) due to excessive fluid within them and multiple interfaces between fluid and soft tissues. The heart appears relatively small within the thorax, compressed by lungs. Both hemidiaphragms are inverted due to hyperexpansion of the lungs. The bronchi are typically dilated, and may be identifiable as distended fluid-filled tubular structures within the lungs. Fetal hydrops may be present.
When bronchial atresia is present, the affected lung is large and homogeneously echogenic (Figure 8.2.2). The heart is displaced to the contralateral side.
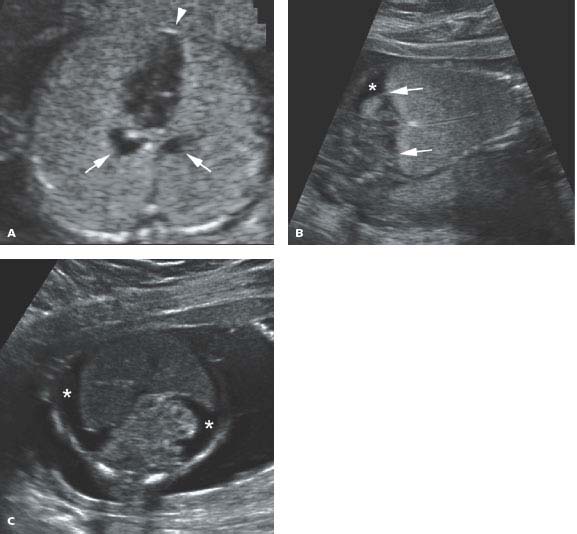
 Figure 8.2.1
Figure 8.2.1
Tracheal atresia. A: Axial image of chest demonstrating markedly enlarged and homogeneously echogenic lungs compressing heart (arrowhead) in the midline. Dilated, fluid-filled bronchi (arrows) are seen posterior to the heart. B: Coronal image showing expanded echogenic lungs with inversion of both hemidiaphragms (arrows). Some ascites (*) is seen in the abdomen. C: Axial image of abdomen showing ascites (*) throughout the abdomen due to obstructed venous return to the heart.
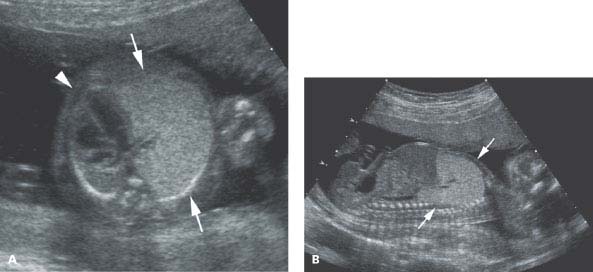
 Figure 8.2.2
Figure 8.2.2
Bronchial atresia. A: Axial image of chest demonstrating large, homogeneously echogenic tissue (arrows) filling left hemithorax, displacing the heart (arrowhead) to the right. B: Sagittal image of left chest showing echogenic tissue (arrows) filling left hemithorax.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree