Measurement of plaque burden is different from measurement of carotid intima-media thickness (IMT). Carotid total plaque area is a stronger predictor of cardiovascular risk than IMT, and in contrast to progression of IMT, which does not predict cardiovascular events, progression of total plaque area and total plaque volume strongly predict cardiovascular events. Measurement of plaque burden is useful in genetic research, and in evaluation of new therapies for atherosclerosis. Perhaps more importantly, it can be used for management of patients. A strategy called “treating arteries instead of treating risk factors” markedly reduces risk among patients with asymptomatic carotid stenosis.
Key points
- •
Carotid plaque burden can be measured by two-dimensional or 3D ultrasound.
- •
Measurements of carotid plaque burden are useful in risk assessment, genetic research, and evaluation of new therapies.
- •
Three-dimensional ultrasound of carotid plaque volume is useful for risk stratification, for evaluating effects of therapy on atherosclerosis, and for managing patients at risk of cardiovascular events, by a strategy that has been called “treating arteries instead of risk factors.”
History of the development of three-dimensional carotid plaque ultrasound
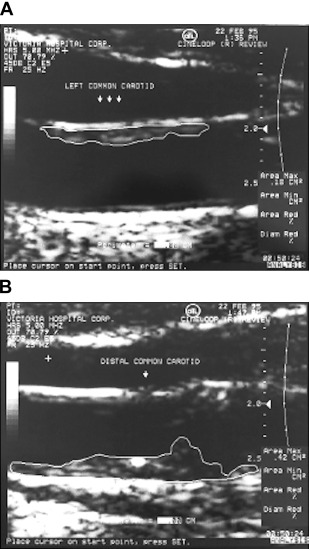
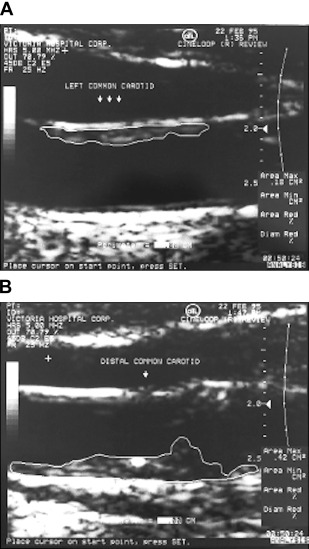
Early applications of ultrasound in the carotid arteries focused on using the Doppler shift to evaluate blood velocity and flow disturbances; our group used implanted ultrasound probes in the carotid arteries to assess effects of antihypertensive drugs on blood velocity and pulsatility, using spectral analysis to evaluate flow patterns. The primary clinical use was for diagnosing carotid stenosis ; we used a primitive device, the Dopscan, to evaluate effects of antihypertensive drugs on turbulence at sites of stenosis. When the author JDS obtained a duplex scanner because it provided spectral analysis to evaluate drug effects on flow disturbances, he realized that it was possible to image and begin to quantify carotid plaque burden. It was Maria DiCicco RVT, in the author’s laboratory, who told JDS that there was software in the scanner that could measure plaque area, and first measured carotid total plaque area (TPA), in 1990. After JDS told Jon Wikstrand about it, Wikstrand’s group published a method in 1992. Then in 1997, TPA was used to study effects of mental stress on atherosclerosis ; Fig. 1 is reproduced from that paper.
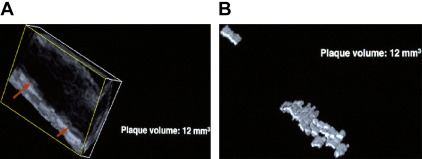
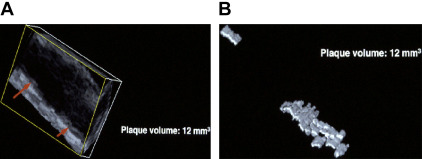
In 1994, around the time that Delcker and Diener published their early work on three-dimensional (3D) ultrasound estimation of plaque volume, Dr Aaron Fenster visited JDS at Victoria Hospital in London, Ontario to ask if he would be interested in using the 3D ultrasound system that Fenster had developed for other purposes to measure 3D carotid plaque volume. JDS visited his laboratory soon after. Fig. 2 shows perhaps the first measurement of plaque volume, in his right carotid artery in 1994 on Fenster’s prototype machine. In 1995 JDS moved to University Hospital and the Robarts Research Institute to collaborate with Dr Fenster, and then later Dr Grace Parraga, to develop 3D ultrasound of carotid plaque.
Topics discussed in this article include measurement of plaque burden, ulceration, echolucency, and plaque texture.
History of the development of three-dimensional carotid plaque ultrasound
Early applications of ultrasound in the carotid arteries focused on using the Doppler shift to evaluate blood velocity and flow disturbances; our group used implanted ultrasound probes in the carotid arteries to assess effects of antihypertensive drugs on blood velocity and pulsatility, using spectral analysis to evaluate flow patterns. The primary clinical use was for diagnosing carotid stenosis ; we used a primitive device, the Dopscan, to evaluate effects of antihypertensive drugs on turbulence at sites of stenosis. When the author JDS obtained a duplex scanner because it provided spectral analysis to evaluate drug effects on flow disturbances, he realized that it was possible to image and begin to quantify carotid plaque burden. It was Maria DiCicco RVT, in the author’s laboratory, who told JDS that there was software in the scanner that could measure plaque area, and first measured carotid total plaque area (TPA), in 1990. After JDS told Jon Wikstrand about it, Wikstrand’s group published a method in 1992. Then in 1997, TPA was used to study effects of mental stress on atherosclerosis ; Fig. 1 is reproduced from that paper.
In 1994, around the time that Delcker and Diener published their early work on three-dimensional (3D) ultrasound estimation of plaque volume, Dr Aaron Fenster visited JDS at Victoria Hospital in London, Ontario to ask if he would be interested in using the 3D ultrasound system that Fenster had developed for other purposes to measure 3D carotid plaque volume. JDS visited his laboratory soon after. Fig. 2 shows perhaps the first measurement of plaque volume, in his right carotid artery in 1994 on Fenster’s prototype machine. In 1995 JDS moved to University Hospital and the Robarts Research Institute to collaborate with Dr Fenster, and then later Dr Grace Parraga, to develop 3D ultrasound of carotid plaque.
Topics discussed in this article include measurement of plaque burden, ulceration, echolucency, and plaque texture.
Measurement of plaque burden and its progression/regression
Measurement of carotid intima-media thickness (IMT) had begun around 1985, first in monkeys and then in human subjects. Having been taught atherosclerosis by Dr Daria Haust (for many years the Editor of Atherosclerosis , and a Professor of Pathology at University of Western Ontario), JDS understood early on that IMT did not truly represent atherosclerosis, and decided to focus on quantification of plaque burden.
In 2002 his group reported that TPA was a strong predictor of cardiovascular risk among patients attending cardiovascular prevention clinics. After adjusting for age, sex, blood pressure, cholesterol, smoking, diabetes, homocysteine, and treatment of blood pressure and cholesterol, patients in the top quartile of TPA had a 3.4 times higher 5-year risk of stroke, death, or myocardial infarction. By quartile of TPA, the 5-year risks were approximately 5%, 10%, 15%, and 20%. Thus TPA was much stronger than a Framingham risk profile in predicting risk. During the first year of follow-up, approximately half the patients had plaque progression, a quarter had regression, and a quarter was stable. Those with plaque progression had twice the risk of events, after adjustment for the panel of risk factors listed previously. Our findings were borne out in the Tromsø study, a population-based study in Northern Norway of more than 6000 participants who were healthy at baseline. That study showed that TPA, but not IMT in the distal common carotid, predicted myocardial infarction and stroke. Added to risk calculation using scores based on risk factors, TPA significantly improves risk prediction. Subsequently it has become apparent in meta-analyses that IMT is only a weak predictor of cardiovascular events, adding little to a Framingham risk score, and that progression of IMT does not predict events. Brook and colleagues found that TPA significantly changed risk prediction and was more predictive of coronary stenosis than IMT, coronary calcium score, or C-reactive protein. Sillesen and colleagues found that carotid plaque burden (an estimated plaque volume assessed by moving the probe along the carotid) was much more closely correlated with coronary calcium score than IMT.
Measurement of Three-Dimensional Plaque Volume
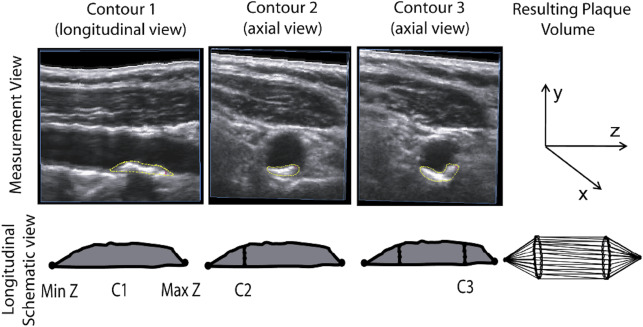
Perhaps the first description of estimation of plaque volume was in a study by Hennerici and Steinke in 1987. Initial measurements of carotid plaque volume were laborious; the method, called disk segmentation, required acquisition of a series of two-dimensional cross-sectional slices captured by translating the ultrasound probe along the carotid artery with a mechanical device, tracing of the plaque area in serial cross-sections of the artery, stepping through the plaque at intervals of 1 to 2 mm, and summing the slices to obtain plaque volume ( Fig. 3 ). Early work specified the reproducibility, reliability, interslice distance, and other technical issues. Ludwig and colleagues in 2008 and papers in 2014 and 2015 reported good reproducibility, similar to results previously reported by Fenster’s group, and also found that reproducibility was better for large plaques than for small ones.
The disk segmentation method was not only laborious; it required 2 to 3 months of training and practice to learn to do it reliably and achieve certification in its use, and some candidates (approximately a third) were not able to perform it reliably: it seemed that perfectionists struggled too much with deciding where to demarcate boundaries, and others were too careless. These issues were the impetus for development of semiautomated and automated methods that would be less operator-dependent, less laborious, and perhaps more reproducible and reliable.
Early semiautomated methods reduced the number of slices of the plaque that required manual input to set the proximal and distal ends of each plaque, and tracing the contour of slices at the midpoint, and 25% and 75% of the length of the plaque, with automated interpolation of the surface of the remainder of the plaque assuming uniform plaque geometry, adjustable by the operator ( Fig. 4 ).
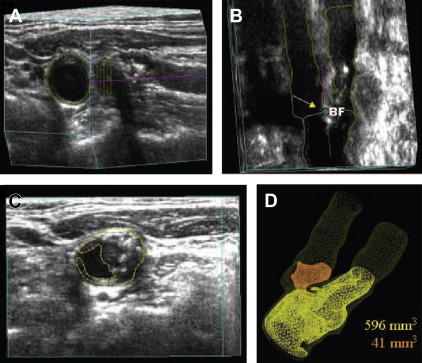
In 2013 an automated method was developed using mechanical movement of the probe along the artery that provided good agreement with manual segmentation by experts ( Fig. 5 ). However, the machine used to translate the ultrasound probe is large and clumsy, and difficult to use in patients with obesity or short necks. A mechanical sweep, obtained by holding the probe in one location and having the angle changed mechanically ( Fig. 6 ), is faster and more convenient. Recently Graebe and colleagues, using the Philips system used by Sillesen and colleagues in the High-Risk Plaque Bioimage Study, showed that the mechanical sweep gave improved reproducibility of plaque volume quantification compared with manual translation of the probe along the artery ( Fig. 7 ).
Progression of Carotid Plaque Volume and Cardiovascular Risk
Progression of TPA was shown to strongly predict the 5-year risk of stroke, myocardial infarction, and death after adjustment for risk factors. Carotid plaques grow along the artery 2.4 times faster than they thicken, so measuring plaque area is more sensitive than measuring thickness alone. Plaques also grow and regress circumferentially, so measuring 3D plaque volume is even more sensitive than measuring area. Carotid 3D plaque volume is also more sensitive to effects of therapy than coronary intravascular ultrasound (IVUS): whereas carotid plaques are focal and can change in three dimensions, coronary plaques are present along the entire length and entire circumference of the artery, so change is reduced to a change in average thickness (ie, a one-dimensional change). In a meta-analysis, Noyes and Thompson concluded that using IMT or IVUS, 2 years would be required to study effects of statins on plaque progression/regression; however, change in TPA or total plaque volume can be done in 3 months.
Progression of plaque volume predicted cardiovascular events in a small sample of prevention clinic patients who had plaque at baseline (N = 323), whereas progression of IMT or TPA did not significantly predict events.
Regression of carotid plaque area was reported in a quarter of patients in 2002 ; by 2010 it was observed in half of patients being followed in the same clinic, probably caused by more intensive medical therapy. In 2005, Ainsworth and colleagues reported that by measuring carotid plaque volume, significant plaque regression with high-dose atorvastatin in patients with carotid stenosis could be shown in only 3 months, in a study comparing only 21 patients randomized to placebo with 17 randomized to atorvastatin. This reduced by two orders of magnitude the sample size and duration of studies to assess new therapies for atherosclerosis compared with IMT. Carotid plaque volume is also significantly more sensitive than IVUS, as reported by Noyes and Thompson in a meta-analysis indicating that patients in studies of antiatherosclerotic therapies should be followed for 2 years to detect effects of therapy. An issue that is little understood is that because carotid plaques are focal, whereas coronary plaques extend throughout the length of the pullback, measuring progression of carotid plaque is more sensitive to effects of therapy than coronary IVUS.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




