Thyroid Cancer and Thyroid Imaging
Michele Brenner
Richard L. Wahl
This chapter is designed to serve as a guide to clinicians seeking to better understand the role of positron emission tomography (PET) in the management of proven thyroid malignancies as well as providing insights on the role of PET in detecting and characterizing primary thyroid disease processes, both malignant and nonmalignant.
The incidence of thyroid cancer ranges between 4 and 12 cases per 100,000 per year, but it is increasing (1,2). Radiation exposure is one of the risk factors for thyroid cancer. Many aspects in the management of this disease are debated. The use of PET adds an additional topic for debate, but its role in management is becoming increasingly well established. The differentiated cancers, papillary and follicular, constitute approximately 90% of thyroid cancers (3) (Figs. 8.8.1 and 8.8.2). Although differentiated thyroid cancers are highly treatable, they require vigilant follow-up. When indicated, initial therapy consists of a total thyroidectomy and radioiodine ablation therapy (4). Despite this, approximately 20% of patients develop recurrences, most within the first 10 years after diagnosis (1,5). After treatment, patients are monitored clinically, with iodine scans and with measurements of serum thyroglobulin. Serum levels of this protein should be undetectable with effective treatment. An increase in serum thyroglobulin over time is suspicious for recurrent or metastatic disease. In 10% to 15% of patients with an elevated thyroglobulin, a diagnostic iodine-131 (131I) whole-body scintigraphy (WBS) to localize disease is negative (6). Poorly differentiated or progressively dedifferentiated thyroid cancer cells often lose the ability to concentrate radioactive 131I. Furthermore, 50 % of these patients, when retreated empirically, will have uptake on posttreatment WBS scan (7), indicating the diagnostic scan was falsely negative. Although false-negative scans could probably be made less frequently by using a higher dose of radioiodine for the scans, this is probably not desirable as “stunning” of the gland could occur, potentially resulting in less-effective treatments. At the same time, these cells often exhibit increased metabolic activity, as evidenced by enhanced glucose uptake (8). The imaging of these poorly differentiated, noniodine-avid metastases and recurrences is problematic. Radioiodine imaging detects well-differentiated recurrences and metastases with a high specificity but only moderate sensitivity. A 2-fluorine-18-fluoro-2-deoxyglucose (18F-FDG) PET may improve on this sensitivity and has been proposed as a molecular imaging modality for these patients.
For many years, 131I, 123I, and technetium-99m (99mTc) pertechnetate have had major roles in evaluating thyroid diseases, both malignant and benign. In areas where 123I costs are not prohibitively high, this tracer has assumed a major role in evaluating the thyroid. Although the positron emitter 124I should be able to do most imaging currently done by other isotopes, and perhaps at a higher quality, it is not yet widely available. Further, the dosimetry of 124I is somewhat problematic in that there is substantial radiation dose deposited by the positron annihilation events and the long, 4-plus-day half-life, coupled with the considerable fraction of decay events associated with gamma ray emissions, which are not imaginable with PET.
Thus, this chapter concentrates on the radiotracer most commonly used in PET thyroid cancer imaging, FDG. The [18F]-FDG PET has already been shown to effectively detect various types of cancer by virtue of their increased glucose metabolism (9).
Metabolism of Glucose
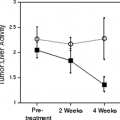
The 18F label allows visualization of physiologic glucose metabolism. FDG, a structural analogue of glucose, is transported across the cell membrane by glucose transporters, phosphorylated to [18F]-FDG-6-PO4 by hexokinase 1, and can no longer diffuse out of the cell. Unlike glucose, FDG cannot enter glycolysis; therefore, it becomes trapped within glucose-metabolizing cells. Normal thyrocytes express glucose transporters and concentrate and phosphorylate glucose. Furthermore, expression of the glucose transporter 1 (GLUT1) gene is increased in some, although not all, human thyroid cancers (10,11,12). Planar gamma camera imaging using a specially modified gamma camera was shown capable of imaging metastatic thyroid cancer in a small study performed in the late 1980s (13). In that small study of three patients it was recognized that there were several different metabolic uptake patterns in these cancers including (a) iodine positive, FDG negative; (b) positive for both tracers, and (c) FDG positive, iodine negative, as well as both negative. The authors also recognized that FDG positive lesions appeared to be more aggressive. In addition, these observers recognized that lesions could vary in uptake patterns within the same patient.
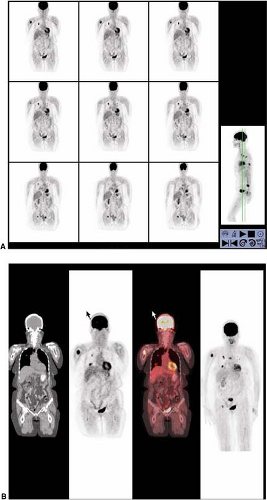
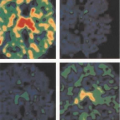
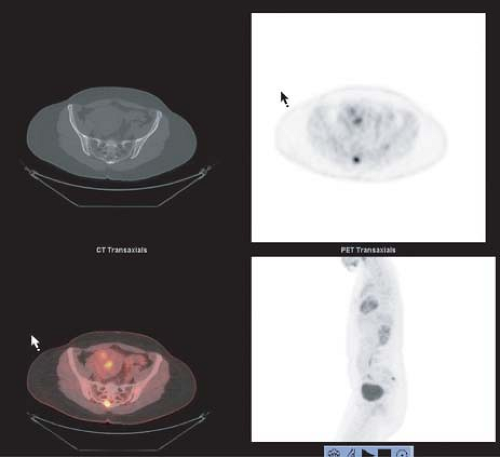 Figure 8.8.1. Metastatic follicular variant of papillary thyroid cancer (to sacrum). Arrow indicates right side of image. |
This same group using a planar gamma camera was able to demonstrate that in the thyroid gland itself, while thyroid cancers did accumulate FDG, several benign thyroid processes also accumulated FDG. Thus, the accumulation of the FDG was not completely specific for malignant thyroid nodules (14). This background is important in considering the emerging role of FDG PET in thyroid cancer imaging.
18-F Fluorodeoxyglucose PET
The role FDG PET (or PET/CT) will play in thyroid cancer remains in evolution. However, the role of FDG PET in the follow-up of patients with well-differentiated thyroid cancer, status post–total thyroidectomy and 131I ablation with increasing thyroglobulin levels and negative 131I whole-body scans is increasingly well established. As indicated, early studies have shown that there can be a flip-flop in uptake patterns in thyroid cancers, with them being first iodine positive, FDG negative, and with progression becoming FDG positive, iodine negative (15). It is these latter tumors, those that are iodine negative, in which FDG PET appears to be most potentially beneficial.
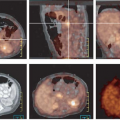
Chung et al. (16) demonstrated that FDG PET was most useful in patients with negative 131I scans, detecting cervical lymph node metastasis in 87.9%, lung metastasis in 27.3%, mediastinal metastasis in 33.3%, and bone metastasis in 9.1%. In a study by Hsu et al. (17) 15 patients with posttherapy local invasive or aggressive differentiated thyroid cancer were evaluated. FDG PET was useful for detecting dedifferentiated lesions and was superior to 131I WBS in detecting residual cervical or mediastinal lesions and suspected small metastatic foci in the lung. FDG PET was inferior to 131I WBS in detecting diffuse lung metastases and distant bone metastases. Early prospective studies in small numbers of patients confirm FDG PET’s usefulness in correctly detecting recurrence or metastases. Despite the heterogeneity of 18F-FDG techniques; FDG PET correctly detected metastatic disease in 60% to 94% of patients and changed management in 50% to 78% (3,18,19,20,21,22,23,24). The reported sensitivity and specificity have varied widely as well. The sensitivity has been reported between 40% and 100% (25), and the specificity between 25% and 95.2% (16,20,26). Two larger studies, Wang et al. (23) and Schulter et al. (24), with 37 and 64 patients, respectively, confirmed the utility of FDG PET to localize residual thyroid cancer lesions and affect management in patients who have negative diagnostic 131I whole-body scans and elevated thyroglobulin levels. The positive predictive value ranged 83% to 92% and the negative predictive value ranged from 25% to 93%. True-positive FDG PET findings were correlated with increasing human thyroglobulin level; FDG PET is most often positive at human thyroglobulin levels of greater than 10 μg/L. Overall, follow-up of these patients should be in conjunction with 131I WBS (1,24) for best overall sensitivity and specificity.
In recent years fusion imaging using PET combined with computed tomography (CT) has been able to increase the diagnostic accuracy, reduce pitfalls, and change therapeutic strategies in a substantial number of patients (1). Nahas et al. (27) conducted a retrospective analysis of 33 patients with suspected recurrent papillary thyroid carcinoma who had undergone PET/CT. In 67% of cases, PET/CT supplied additional information that altered or confirmed the management plan. PET/CT correlated correctly with histopathological findings in 25 of 36 distinct anatomical sites, with an accuracy of 70%. The sensitivity of PET/CT in identifying recurrence was found to be 66%, with a specificity of 100%, a positive predictive value of 100%, and a negative predictive value of 27%. Therefore, a negative finding on PET/CT is not sufficiently reliable to preclude further investigation and treatment. Importantly, in this study a positive PET/CT is a powerful tool for predicting exact
locations of recurrent papillary thyroid cancer, thus making it a generally reliable guide for surgical planning. Furthermore, the modality was most useful in patients who had average thyroglobulin levels greater than 10 ng/mL and when the tumor no longer concentrated radioactive iodine.
locations of recurrent papillary thyroid cancer, thus making it a generally reliable guide for surgical planning. Furthermore, the modality was most useful in patients who had average thyroglobulin levels greater than 10 ng/mL and when the tumor no longer concentrated radioactive iodine.
Similar findings were reported by Shammas et al. (9) in a prospective study, with [18F]-FDG PET/CT of 61 consecutive patients who had elevated thyroglobulin levels or a clinical suspicion of recurrent disease after total thyroidectomy followed by radioiodine ablation. Clinical management changed for 27 (44%) of 61 patients, including surgery, radiation therapy, or chemotherapy. Their accuracy (73.8%) was equally high. Furthermore, [18F]-FDG PET/CT can provide precise anatomic localization of recurrent or metastatic thyroid carcinoma, leading to improved diagnostic accuracy, and can guide therapeutic management. In two patients, increased 18F-FDG uptake identified a second primary malignancy. Although not as robust, they report a specificity of 82.4%, with four false positives, and a sensitivity of 68.4%, with 12 false-negative patients. Similarly, the sensitivities of 18F-FDG PET/CT at serum thyroglobulin levels of less than 5, 5 to 10, and more than 10 ng/mL were 60%, 63%, and 72%, respectively. A negative finding on PET/CT is not sufficiently reliable to preclude further investigation and treatment. It is also commonly the case that the number of FDG avid foci seen at PET is fewer than the number of lymph nodes containing tumor removed surgically.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree