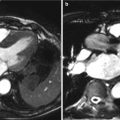
Fig. 4.1
Current balloon-expandable transcatheter heart valves: Edwards SAPIEN THV (a) schemata and (b) following deployment with aortography and Edwards SAPIEN XT THV (c) schemata and (d) following deployment with aortography
The SAPIEN™ THV utilizes a stainless steel frame and is available in two sizes, with external diameters of 23 and 26 mm and heights of 14.3 and 16.1 mm, respectively, once fully deployed. The newer SAPIEN XT™ THV consists of a cobalt chromium frame; the strength of the alloy is greater allowing a reduction in strut thickness with fewer stent struts and therefore a lower-profile device for delivery. As a result, radial strength may be lower. It is available in four sizes, with external diameters of 20, 23, 26, and 29 mm and heights of 13.5, 14.3, 17.2, and 19.1 mm, respectively (Fig. 4.2).
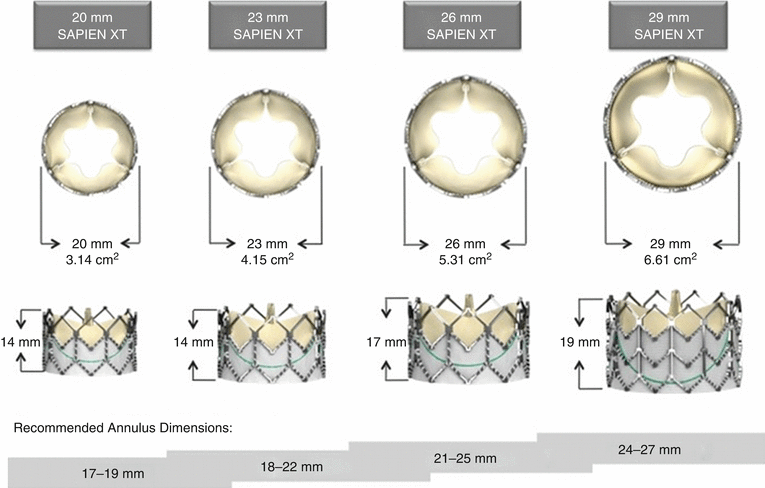

Fig. 4.2
Edwards SAPIEN XT dimensions and recommended annulus sizes
The current manufacturer’s recommendations are to select device size according to two-dimensional long-axis TEE measurements of the aortic annulus, although emerging evidence by CT has challenged the accuracy of such measurements. The 23 mm valve is recommended for use in patients with annulus diameters of 18–22 mm and the 26 mm in patients with annulus diameters of 21–25 mm, while the 29 mm SAPIEN XT valve is recommended for use in patients with annulus diameters of 24–27 mm.
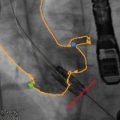
The Edwards THVs are generally implanted utilizing a transarterial or transapical approach. For transarterial implantation (Fig. 4.3), the SAPIEN THV is compressed onto the Retroflex 3 delivery system that is introduced into the femoral artery via a 22 or 24 F sheath for the 23 and 26 mm valves, respectively. The newer-generation SAPIEN XT is crimped onto the lower-profile Novaflex delivery system, resulting in lower sheath sizes of 16, 18, and 20 F for the 23, 26, and 29 mm valves, respectively (Fig. 4.4). This lower-profile delivery system has expanded the number of patients eligible for the transfemoral approach who would otherwise have been excluded due to small vessel diameter and has resulted in a significant reduction in vascular complications [14]. Recommended minimal luminal diameters of the iliofemoral system are demonstrated in Table 4.1 for each valve and sheath size. These recommendations, however, do not take into account vessel calcification, atheroma, and tortuosity. In the absence of severe disease, a relatively compliant artery may accommodate a slightly larger sheath size.
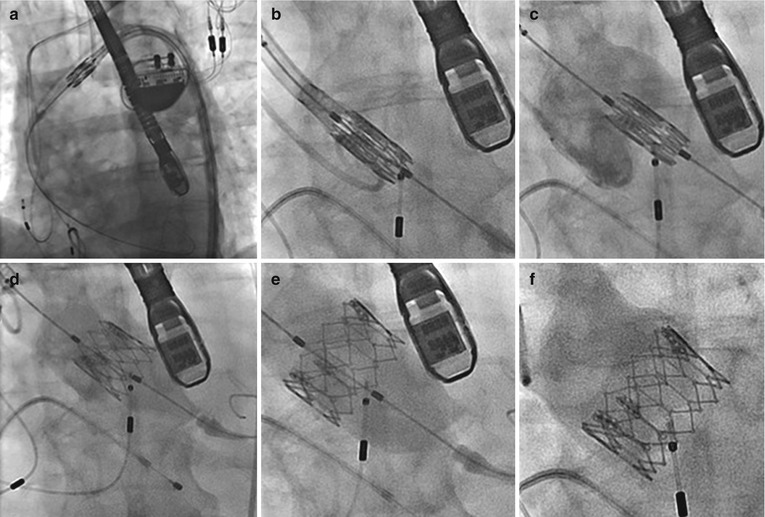
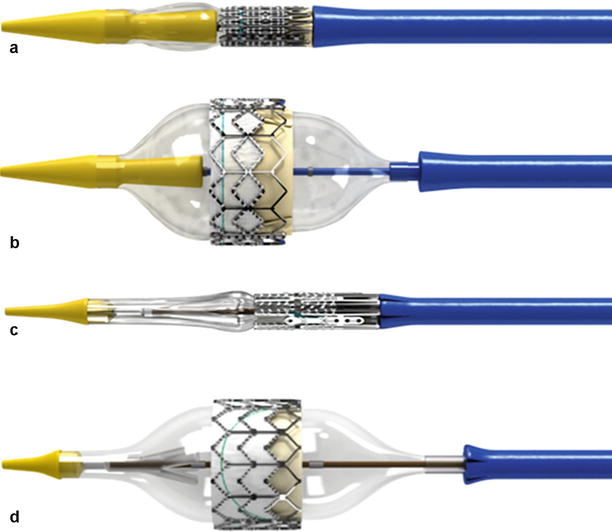

Fig. 4.3
Transfemoral delivery of Edwards balloon-expandable transcatheter heart valve. (a) Passage of the valve across the aortic arch. (b) Crossing the native aortic valve. (c) Positioning of the valve utilizing aortography and transesophageal echocardiography. (d) Partial balloon expansion of the transcatheter heart valve. (e) Full balloon expansion of the transcatheter heart valve. (f) Post implantation aortography

Fig. 4.4
Edwards balloon-expandable delivery systems. Retroflex 3 delivery system with (a) crimped valve and (b) balloon-expanded valve and Novaflex + delivery system with (c) valve crimped and (d) balloon-expanded valve
Table 4.1
Edward SAPIEN and SAPIEN XT sizes, sheath dimensions, and recommended minimum artery diameter
SAPIEN | SAPIEN XT | ||||||
|---|---|---|---|---|---|---|---|
THV size (mm) | 23 | 26 | 20 | 23 | 26 | 29 | |
Sheath ID (French) | 22 | 24 | 16 | 16 | 18 | 20 | |
Sheath OD (mm) | Unexpanded | 8.4 | 9.2 | 6.7 | 6.7 | 7.2 | 8 |
Expanded with valve | – | – | 8.9 | 8.9 | 8.9 | 9.9 | |
Recommended minimum artery diameter (mm) | 7 | 8 | 6 | 6 | 6.5 | 7 | |
For the transapical approach, the THV is crimped onto the balloon and delivered using the shorter Ascendra delivery system for the SAPIEN THV and the Ascendra 2 delivery system for the SAPIEN XT.
CoreValve
The CoreValve ReValving System™ (Medtronic Inc., Irvine, USA) consists of porcine pericardial leaflets mounted within a self-expanding nitinol frame (Fig. 4.5). The current-generation device is compressed within its Accutrak™ delivery catheter and introduced through an 18 French sheath. It is manufactured in 26, 29, and 31 mm outer diameters, as measured at the ventricular part of the frame, recommended for use in patients with annulus diameters of 20–23, 23–27, and 26–29 mm, respectively. The frame has an overall length of up to 55 mm, and in addition to anchoring at the level of the annulus and valve leaflets, the CoreValve extends superiorly to anchor in the ascending aorta.
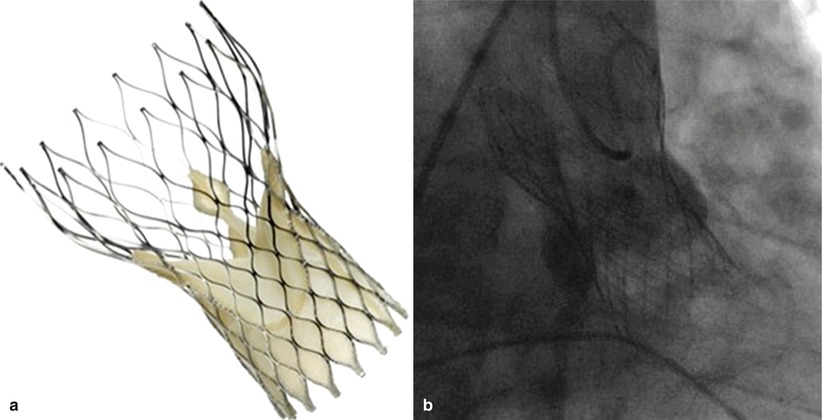

Fig. 4.5
CoreValve ReValving System (a) schemata and (b) following deployment with aortography
Careful evaluation of the aortic root and ascending aorta, in addition to that of the aortic annulus, is required to avoid compromise of the coronary artery ostia and avoid valve embolization (Fig. 4.6). It is recommended that a coronary ostial height of 15 mm is required to ovoid coronary obstruction if the 12 mm skirt is deployed too aortic. Additionally, the sinus of Valsalva width needs to be greater than 27 mm for the 26 mm device and greater than 29 mm for the larger devices, to ensure a space surrounding the central waist of the device, to house the displaced native valve leaflets. The recommended maximum diameter of the ascending aorta is 40, 43, and 43 mm for the 26, 29, and 31 mm devices, respectively, to allow for satisfactory anchoring of the device at its upper portion (Table 4.2). Care must be taken in those patients with bypass grafts to ensure the device does not interfere with graft ostia.
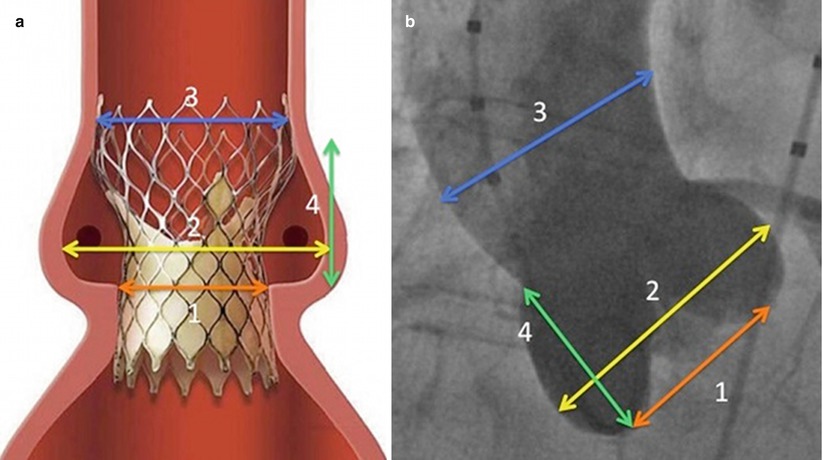

Fig. 4.6
Aortic root and annulus measurements required for CoreValve demonstrated on (a) schemata and (b) aortography. The required measurements include (1) aortic annulus diameter, (2) sinus of Valsalva width, (3) ascending aorta width, and (4) sinus of Valsalva height
Table 4.2
CoreValve dimensions and manufacturer’s recommendations
26 mm | 29 mm | 31 mm | ||
|---|---|---|---|---|
Annulus diameter (mm) | 20–23 | 23–27 | 26–29 | |
Dimensions of expanded valve (mm) | Inflow | 26 | 29 | 31 |
Constriction | 22 | 23 | 23 | |
Outflow | 40 | 44 | 44 | |
Length | 55 | 53 | 52 | |
Ascending aorta diameter (mm) | ≤40 | ≤43 | ≤43 | |
Sinus of Valsalva | Width (mm) | ≥27 | ≥29 | ≥29 |
Height (mm) | ≥15 | ≥15 | ≥15 | |
Sheath ID (French) | 18 | 18 | 18 | |
Recommended minimum artery diameter (mm) | ≥6 | ≥6 | ≥6 | |
Since the CoreValve is self-expanding, it has the potential benefit of partial retrieval if positioned incorrectly (Fig. 4.7). Disadvantages when compared with the SAPIEN valves include a higher rate of atrioventricular block, pacemaker insertion, paravalvular regurgitation, and the potential late consequences of covering the coronary ostia.
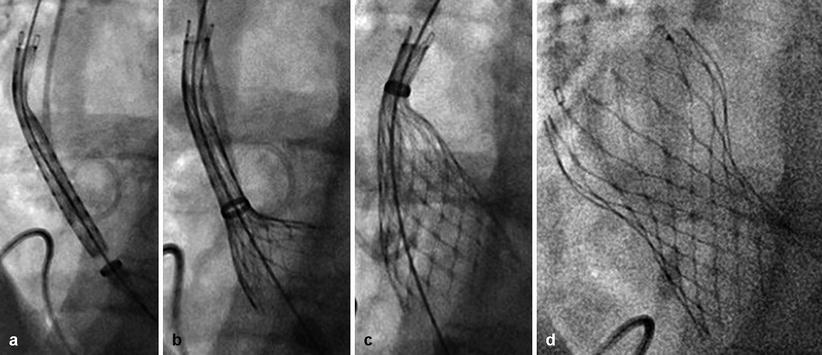

Fig. 4.7
Delivery of the CoreValve ReValving System: (a) Crossing the native aortic valve. (b) Retraction of the outer sheath. (c) Partial release of the transcatheter heart valve at which point it is still possible to retrieve the valve. (d) Full expansion of the prosthesis extending from just below the insertion of the native leaflets to the supracoronary aorta
Newer Valves
Several newer transcatheter heart valves are under evaluation, including the Lotus™ valve (Boston Scientific Inc., USA), Portico™ (St Jude Medical Inc., USA), Engager™ (Medtronic Inc., USA) [15], JenaClip™ (JenaValve Inc., DE) [16, 17], Acurate (Symetis Inc., CH), and Direct Flow™ valves (Direct Flow Medical Inc., USA) (Fig. 4.8) [18, 19]. Generally, these next-generation valves are self-expanding and include features that may facilitate valve positioning and improve annular sealing, with lower-profile delivery systems to accommodate smaller vessel diameters. These valves are currently under clinical evaluation and it remains unclear whether outcomes will be inferior, comparable, or superior to current-generation THVs.


Fig. 4.8
Newer transcatheter heart valves under evaluation: (a) Lotus valve (Boston Scientific Inc. USA), (b) Portico valve(St. Jude Medical Inc. USA), (c) Engager valve(Medtronic Inc. USA), (d) JenaClip valve (JenaValve Inc. DE), (e) Acurate valve (Symetis Inc. Ch), and (f) Direct Flow valve (Direct Flow Medical Inc. USA)
Patient Selection and Evaluation
Although feasible in most patients with severe aortic stenosis, TAVR is generally utilized in patients not suitable for surgical AVR, who are likely to derive functional and survival benefit. The evaluation of potential TAVR patients is a complex process involving a multidisciplinary team approach; key members of the team include the primary cardiologist, interventional cardiologist, cardiothoracic surgeon, vascular surgeon, cardiac anesthesiologist and perfusionist, echocardiographers, and cardiac imaging specialists, as well as specialized nursing. This multidisciplinary approach is also applied during performance of the procedure and postoperative care of the patient [20].
“High-risk” surgical patients are often defined as having a Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) at 30 days of greater than 10 % or logistic EuroSCORE of greater than 20 % (Box 4.1). These risk calculators are often limited, however, and do not account for several pertinent clinical risk factors, such as previous coronary artery bypass grafting with patent grafts, porcelain aorta, previous chest radiotherapy, severe lung disease, and liver cirrhosis.
A comprehensive evaluation involving transthoracic echocardiography, coronary angiography, aortic angiography, and CT is required prior to TAVR to assess the aortic annulus dimensions and geometry, access site, and approach. Transthoracic echocardiography is used to assess valve morphology, transaortic gradient, valvular regurgitation, calcification, orifice area, and other concomitant cardiac pathologies. It is also used as a screening tool to evaluate aortic annulus dimensions prior to device selection. Final valve sizing, however, is usually dependant on intraoperative two-dimensional long-axis TEE measurements. Three-dimensional annular sizing with 3-D echocardiography and CT is emerging as a potentially more accurate way of sizing the aortic annulus, the latter of which is utilized by many operators including our own. Generally, the implanted valve should be slightly larger than the annulus size to minimize the risk of malapposition, paravalvular leak, and valve embolization associated with undersizing. Excessive oversizing, however, may result in incomplete stent expansion potentially affecting valve hemodynamics or aortic root injury or rupture.
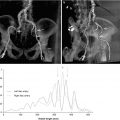
Arterial access is assessed with the combination of invasive angiography and contrast-enhanced CT (Fig. 4.9). As discussed above, the evaluation of arterial dimensions and the presence or absence of atheroma, calcification, and tortuosity is fundamental in the assessment of the TAVR patient in minimizing potential major vascular complications.
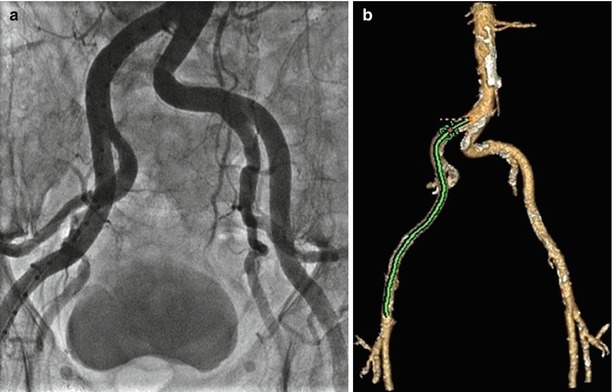

Fig. 4.9
Iliofemoral assessment with (a) angiography and (b) MDCT
The aortic root is assessed using invasive angiography and contrast MDCT to evaluate root and valvular calcification, left main height from the left coronary cusp insertion (due to risk of coronary obstruction), and technical issues related to each valve type and delivery system. Left and right heart catheterizations are also performed to assess the presence of pulmonary hypertension and coronary ischemia and the need for revascularization prior to TAVR. Percutaneous revascularization may be considered as a staged procedure in the presence of a large ischemic burden; however, in the majority of cases, this is not required. Measurement of the aortic transvalvular pressure gradient and calculation of aortic valve orifice area may be useful in cases of aortic stenosis of uncertain severity on transthoracic echocardiography.
Ideally, TAVR should be performed in regional centers of excellence with a dedicated heart valve program and high procedural volumes [20]. The procedure may be undertaken in a cardiac catheterization laboratory with modifications or in a hybrid operating room equipped with high-quality fluoroscopic imaging; the facilities need to be large enough to accommodate sophisticated X-ray imaging integrated with echocardiography, cardiopulmonary bypass and intra-aortic balloon pump machines, and anesthesia equipment, with surgical sterility standards mandatory.
Box 4.1 Variable Within the Society of Thoracic Surgery (STS) and EuroSCORE
STS for Aortic Value Replacement | EuroSCORE |
|---|---|
Age | Age |
Gender | Gender |
Race | Renal impairment |
Weight | Extracardiac arteriopathy |
Height | Poor mobility |
Diabetes | Previous cardiac surgery |
Renal function | Chronic lung disease |
Hypertension | Active endocarditis |
Endocarditis | Critical pre-operative state |
Chronic lung diseases | Diabetes on insulin |
Immunosuppresive therapy | NYHA heart failure class |
Peripheral vascular disease | CCS class 4 angina |
Cerebrovascular disease | LV function |
Prior CABG | Recent myocardial infarction within 90 days |
Prioi valve surgery | Pulmonary hypertension |
Previous other cardiac – PCI | Operation urgency |
Prior myocardial infarction | Surgical weight |
Cardiac presentation (symptoms or ACS) | Surgery on thoracic aorta |
Congestive heart failure | |
Cardiogenic shock | |
Resuscitation | |
Arrhythmia | |
Preoperative inotropes | |
Number of diseased coronary vessels | |
Left main disease | |
Ejection fraction | |
Aortic stenosis | |
Mitral stenosis | |
Aortic regurgitation | |
Mitral regurgitation | |
Tricuspid regurgitation | |
First or reoperative surgery | |
Status of procedure (e.g., elective versus urgent) | |
Intraoperative intraaortic balloon pump
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|