Study
Approach
TAVI System
Mean age
Logistic EuroSCORE %
Periprocedure/30-day stroke %
30-day mortality
1-year stroke%
1-year survival
Piazza et al. [6]
Europe (51 sites)
n = 646
TF: 646
CoreValve®
TF: 81.0
TF: 23.1 ± 13.8
TF: 1.9
TF: 8.0
N/A
N/A
Rodés-Cabau et al. [7]
Canada (6 sites)
n = 339
TF: 162
TA: 177
Cribier-Edwards: 57
Edwards SAPIEN®: 275
Edwards SAPIEN XT®: 7
TF: 83
TA: 80
Overall: 27.7 ± 16.3
TF: 25.8 ± 14.9
TA: 29.4 ± 17.2
Overall: 2.3
TF: 3.0
TA: 1.7
Overall: 10.4
TF: 9.5
TA: 11.3
N/A
Overall: 76
TF: 75
TA: 78
Thomas et al. [7]
Europe (32 sites)
n = 1038
TF: 463
TA: 575
Edwards SAPIEN®
TF: 81.7
TA: 80.7
TF: 25.7 ± 14.5
TA: 29.1 ± 16.3
Overall: 2.5
TF: 2.4
TA: 2.6
Overall: 8.5
TF: 6.3
TA: 10.3
Overall: 5.5
Overall: 76.1
TF: 81.1
TA: 72.1
Eltchaninoff et al. [9]
France (16 sites)
n = 244
TF Edwards: 95
TA Edwards: 71
TF CoreValve®: 66
SC CoreValve®: 12
Edwards SAPIEN®: 166
CoreValve®: 78
Overall: 82.3
TF Edwards: 83.2
TA Edwards: 82.1
TF CoreValve®: 82.5
SC CoreValve®: 75.5
Overall: 25.6 ± 11.4
TF Edwards: 25.6 ± 11.3
TA Edwards: 26.8 ± 11.6
TF CoreValve®: 24.7 ± 11.2
SC CoreValve®: 24.6 ± 14.5
Overall: 3.6
TF Edwards: 4.2
TA Edwards: 2.8
TF CoreValve®: 4.5
SC CoreValve®: 0
Overall: 12.7
TF Edwards: 8.4
TA Edwards: 16.9
TF CoreValve®: 15.1
SC CoreValve®: 8.3
N/A
N/A
Zahn et al. [11]
Germany (22 sites)
n = 697
TF: 644
SC: 22
TA: 26
Tao: 5
Edwards SAPIEN®: 109
CoreValve®: 588
Overall: 81.4
Overall: 20.5 ± 13.2
Overall: 2.8
Overall: 12.4
N/A
N/A
Bosmans et al. [12]
Belgium (15 sites)
n = 328
TF/TA Edwards: 187
TF CoreValve®: 141
Edwards SAPIEN®: 187
CoreValve®: 141
Overall: 83
TF/TA Edwards: 83
TF CoreValve®: 82
Overall: 28 ± 16
TF/TA Edwards: 30 ± 16
TF CoreValve®: 25 ± 15
Overall: 5.0
TF/TA Edwards: 5.0
TF CoreValve®: 4.0
Overall: 11.0
TF/TA Edwards: 12.0
TF CoreValve®: 11.0
N/A
TF Edwards : 82
TF/TA Edwards: 63
TF CoreValve®: 79
Tamburino et al. [13]
Italy (14 sites)
n = 663
TF: 599
SC: 64
CoreValve®
Overall: 81
Overall: 23.0 ± 13.7
Overall: 1.2
Overall: 5.4
Overall: 2.5
Overall: 85
Moat et al. [14]
United Kingdom (25 sites)
N = 870
TF: 599
Other approaches: 271
Edwards SAPIEN®: 410
CoreValve®: 452
Overall: 81.9
TF: 81.7
Other routes: 82.3
CoreValve®: 81.3
Edwards: 82.6
Overall:
18.5 (11.7–27.9)
TF: 17.1 (11, 25.5)
Other routes:
21.4 (14.4, 33.6)
CoreValve®:
18.1 (11.1, 27.9)
Edwards:
18.5 (12.4, 27.7)
Overall: 4.1
TF: 4.0
Other routes: 4.1
CoreValve®: 4.0
Edwards: 4.2
Overall: 7.1
TF: 5.5
Other routes: 10.7
CoreValve®: 5.8
Edwards: 8.5
N/A
Overall: 78.6
TF: 81.5
Other routes: 72.3
CoreValve®: 78.3
Edwards: 79.4
Gilard et al. [15]
France (34 sites)
n = 3195
TF: 2361
TA: 567
SC: 184
Other approaches: 83
Edwards SAPIEN®: 2107
CoreValve®: 1043
Other systems : 45
Overall: 82.7
TF: 83
TA: 81.5
SC: 82.2
Edwards SAPIEN®: 82.9
CoreValve®: 82.3
Overall: 21.9 ± 14.3
TF: 21.2 ± 14.7
TA: 24.8 ± 14.7
SC: 20.3 ± 15.2
Edwards SAPIEN®: 22.2 ± 14.3
CoreValve®:
21.3 ± 14.3
Overall: 3.4
Overall: 9.7
TF: 8.5
TA: 13.9
SC: 10.1
Edwards SAPIEN®: 9.6
CoreValve®: 9.4
Overall: 4.1
Overall: 24.0
TF: 21.7
TA: 32.3
SC: 25.1
Edwards SAPIEN®: 24.0
CoreValve®: 23.7
Wendler [16]
Europe (94 sites)
n = 2600
TF Edwards: 1694
TA Edwards: 906
Edwards
SAPIEN XT®
TF: 81.9
TA: 80.2
TF: 19.9 ± 12.0
TA: 21.8 ± 13.8
TF: 2.3
TA: 2.1
TF: 4.3
TA: 9.9
NA
NA
Linke et al. [17]
Europe, Asia, South America (44 sites)
n = 1015
CoreValve®: 1015
CoreValve®
Overall: 81
Overall: 19.2 ± 12.4
Overall: 2.9
Overall: 4.5
NA
NA
Stroke diagnostic criteria |
Rapid onset of a focal or global neurological deficit with at least one of the following: |
Change in level of consciousness, hemiplegia, hemiparesis, numbness or sensory loss affecting one |
Side of the body, dysphasia or aphasia, hemianopia, amaurosis fugax, or other neurological signs or |
Symptoms consistent with stroke |
Duration of a focal or global neurological deficit ≥24 h; OR <24 h, if therapeutic intervention(s) were performed (e.g., thrombolytic therapy or intracranial angioplasty); OR available neuroimaging documents a new hemorrhage or infarct; OR the neurological deficit results in death |
No other readily identifiable nonstroke cause for the clinical presentation (e.g., brain tumor, trauma, infection, hypoglycemia, peripheral lesion, pharmacological influences) |
Confirmation of the diagnosis by at least one of the following: |
Neurology or neurosurgical specialist |
Stroke definitions |
Transient ischemic attack: |
New focal neurological deficit with rapid symptom resolution (usually 1–2 h), always within 24 h |
Neuroimaging without tissue injury |
Stroke: |
(diagnosis as above, preferably with positive neuroimaging study) |
Minor—Modified Rankin score 2 at 30 and 90 days† |
Major—Modified Rankin score 2 at 30 and 90 days |
The PARTNER trial, to date, remains the only prospective randomized trial of TAVR. PARTNER included two differentiated cohorts of patients: (1) those considered to be nonoperable (i.e., comorbidities leading to a predicted risk of 50 % or more, of either death by 30 days after surgery or a serious irreversible morbidity; patients with comorbidities leading to a life expectancy <1 year were excluded, n = 358) [19] and (2) those considered to be at high surgical risk (i.e., predicted risk of operative mortality ≥15 % as determined by the trial site surgeon and cardiologist; guideline = STS score ≥10, n = 699) [20]. The Edwards SAPIEN valve was used in all cases. The PARTNER trial showed, in the nonoperable cohort, the superiority of TAVR vs. medical treatment with balloon aortic valvuloplasty [BAV] (mortality at 1-year follow-up of 30.7 % vs. 50 %, p < 0.001) and the non-inferiority of TAVR vs. SAVR in the high-risk cohort (mortality at 1-year follow-up of 24.2 % vs. 26.8 %, p = 0.44). However, the trial revealed a higher rate of cerebrovascular events in the TAVR group with respect to the medical treatment and SAVR groups. A summary of the results regarding the CVE in the PARTNER trial at 30-day and at 1-year follow-up is shown in Fig. 20.1.
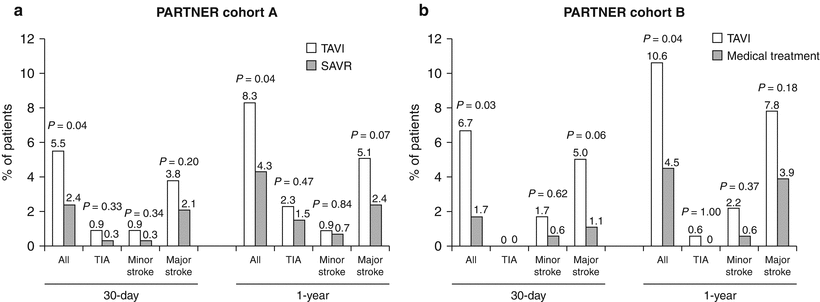

Fig. 20.1
Cerebrovascular events in the PARTNER trial at 30-day and at 1-year follow-up in the nonoperable cohort (a) and the high-risk cohort (b)
The incidence of late (>30 days) ischemic stroke after TAVR (~2 %/year) was similar to that observed after SAVR and medical treatment in a similar population of elderly and high-risk patients with severe aortic stenosis, strongly suggesting the lack of a late prothrombotic effect associated with transcatheter valves [19, 20]. A substudy of the PARTNER trial showed that a higher atherosclerotic burden and previous CVE were the factors associated with late CVE after TAVR [21]. On the other hand, an excess of late hemorrhagic strokes was observed in the TAVR group compared with the medical treatment group in the PARTNER trial [22], highlighting the potential importance of excessive antithrombotic treatment to these late events after TAVR.
Cerebral Ischemic Defects Following TAVR
Clinically silent cerebrovascular injury resulting from TAVR has come to the fore following a series of studies evaluating cerebral ischemic events using diffusion-weighted magnetic resonance imaging (DW MRI) (Table 20.3, Figs. 20.2 and 20.3). An initial study by Kahlert et al. [23] compared 32 patients undergoing transfemoral (TF) TAVR (22 with the Edwards SAPIEN valve [Edwards Lifesciences Inc., Irvine, CA] and 10 with the CoreValve [Medtronic, Minneapolis, MN]) to historical SAVR controls (n = 21) (Table 20.3). Patients underwent DW MRI at baseline, a mean of 3.4 (2.5–4.4) days, and again at 3 months post-index procedure. All patients were assessed by an experienced neurologist using both the National Institutes of Health Stroke Scale (NIHSS) and the Mini Mental State Examination (MMSE). Successful valve implantation was achieved in all TAVR patients with no obvious adverse neurologic event during the periprocedural period. No change was noted in the NIHSS scale or MMSE score of any of these patients. Repeat DW MRI post TAVR however demonstrated new hyperintense lesions in 86 % of the Edwards SAPIEN and 80 % of CoreValve TAVR patients. There were significantly fewer patients with new lesions in the control SAVR group (48 %, p = 0.016). These new lesions were typically identified in both hemispheres reflecting a probable embolic cause. Among individuals with new cerebral lesions, lesion size was significantly smaller in the TAVR groups compared with the SAVR patients. At 3 months, no new DW MRI findings or changes in clinical or neurocognitive testing were noted. Indeed, the authors reported that 80 % of post-TAVR lesions had no residual signal change on repeat DW MRI at the 3-month interval.
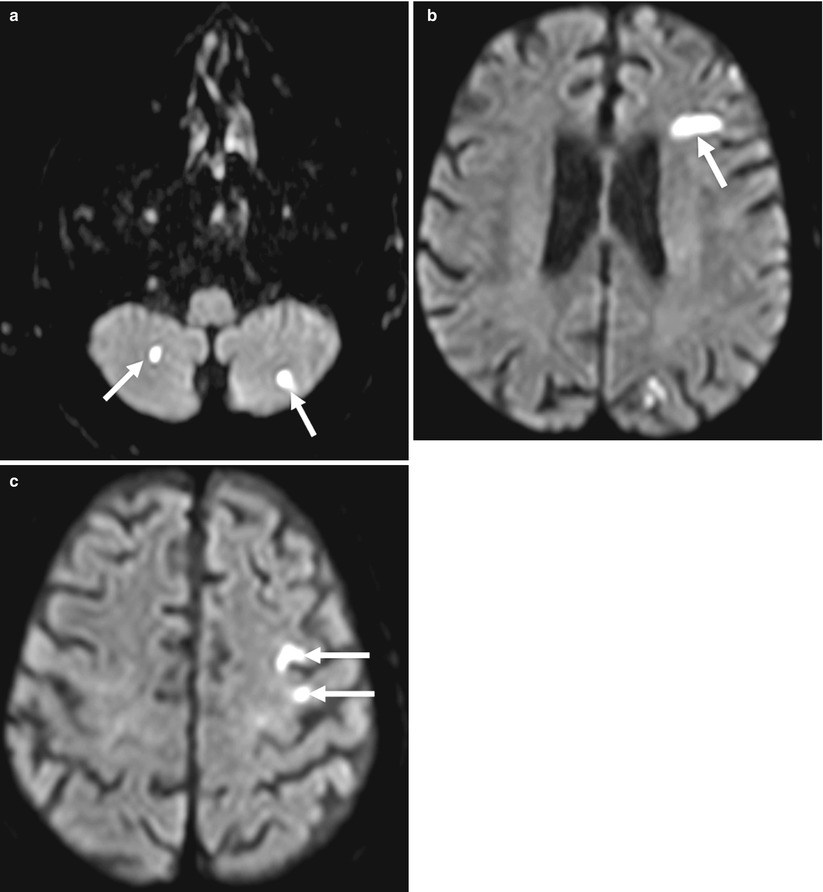
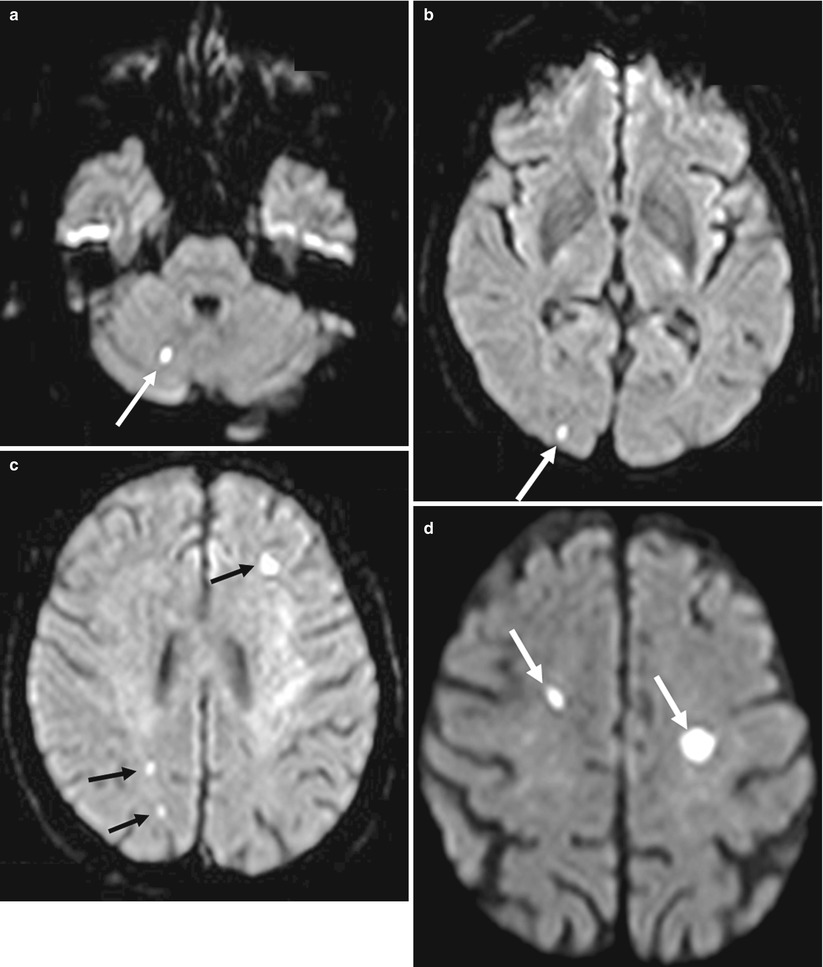
Table 20.3
Diffusion-weighted magnetic resonance imaging studies post transcatheter aortic valve implantation
Study | n | Valve type | Approach | Ischemic defects | Median number of lesions | Cognitive/neurologic assessment | Cognitive/neurologic assessment results | Stroke |
|---|---|---|---|---|---|---|---|---|
Kahlert et al. [23] | 32 | Edwards SAPIEN (n = 22) CoreValve (n = 10) | TF | Edwards: 86 % CoreValve: 80 % | Edwards: 4 (2.1–6.0) CoreValve: 2.6 (0.3–4.9) | NIHSS, MMSE, mRS | No change | Overall: 0 % |
Ghanem et al. [24] | 22 | CoreValve | TF | 73 % | 2.5 (1.0–5.5) | NIHSS, NSE | Neurologic impairment: 3 (10 %) | 3.6 % |
Rodés-Cabau et al. [26] | 60 | Edwards SAPIEN | TF (n = 29) TA (n = 31) | TF: 66 % TA: 71 % | Overall: 3 (2–8) TF: 3 (1–7) TA: 4 (2–9) | NIHSS, MMSE | No change | Overall: 3.3 % TF: 1.6 % TA: 1.6 % |
Fairbairn et al. [27] | 31 | CoreValve | TF | 77 % | 2 (1–5) | NIHSS | No change | 6.0 % |
Arnold et al. [25] | 25 | Edwards SAPIEN | TA | 68 % | N/A | Standardized clinical assessment | Neurologic impairment: 5 (20 %) | 4.0 % |

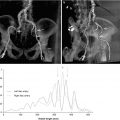
Fig. 20.2
Diffusion-weighted magnetic resonance imaging (DW MRI) images following transapical transcatheter aortic valve implantation (TA TAVI) in an 83-year-old patient showing multiple acute ischemic lesions in the left and right cerebellum (a, white arrows) and left frontal territory (b and c, white arrows) (Reproduced from Rodés-Cabau et al. with the permission of author and publisher)

Fig. 20.3
Diffusion-weighted magnetic resonance imaging (DW MRI) images following transfemoral transcatheter aortic valve implantation (TF TAVI) in an 86-year-old patient showing multiple acute ischemic lesions in the right cerebellum (a, white arrow), right occipital territory (b, white arrow), left frontal and right parietal territories (c, black arrows), and left and right frontal superior territories (d, white arrows) (Reproduced from Rodés-Cabau et al. with the permission of author and publisher)
Ghanem et al. [24] performed a pilot study involving 22 patients to prospectively assess clinically silent and overt cerebral embolic events in the setting of TF TAVR. Cerebral DW MRI was carried out before, within 3 days post and at 3 months after valve implantation. Three patients (10 %) had a new neurological abnormality on examination post TAVR, but symptoms resolved in two. At the time of the first post-TAVR neuroimaging study, 72.7 % of patients had new ischemic lesions compared to baseline.
It had been suggested that the transapical (TA) approach may potentially minimize cardioembolic event rates as this method minimizes the need for extensive catheter manipulation within the aortic arch. Arnold et al. [25] performed a similar assessment using cerebral DW MRI in 25 patients undergoing TA TAVR with the Edwards SAPIEN system. Valve implantation was successful in all patients; however, neurological abnormalities were evident post procedure on clinical examination in five patients, one of who developed a major stroke. Analysis of DW MRI revealed new embolic lesions in 68 % of the patients, with more than one lesion in the majority. In those patients with new lesions, the posterior circulation territory was involved in greater than 50 % of cases. Rodés-Cabau et al. [26] compared the incidence of acute cerebral ischemic injury in those undergoing TAVR (employing the Edwards SAPIEN or SAPIEN XT valve) via the TF and the TA approach. In this multicenter study, 60 patients (TF: 29, TA: 31) underwent cerebral DW MRI prior to and within 6 days following TAVR. Additional neurologic and cognitive evaluation was performed at the same intervals as neuroimaging, using the NIHSS and MMSE tests. One patient in each group had clinical evidence of a stroke within 24 h of valve insertion. Up to 68 % of patients had new ischemic lesions on DW MRI post TAVR with a median number of three lesions per patient (range 1–36). Of those with new lesions, 76 % were multiple and 73 and 66 % involved both cerebral hemispheres and circulation (anterior and posterior) territories, respectively. Of note, most (91 %) lesions were small (<1 cm). There were no significant differences between groups with regard to the incidence of new ischemic lesions between the TF (66 %) and TA (71 %) approaches. Finally, no interval changes in NIHSS and MMSE test scores were recorded following TAVR.
Fairbairn et al. [27] examined quality of life data in addition to performing DW MRI pre and post TAVR with the CoreValve system in 31 AS patients. Two (6 %) patients were diagnosed clinically with stroke as a consequence of the procedure. DW MRI confirmed up to 26 new ischemic lesions in both of these. Overall, new cerebral ischemic lesions were seen in 24 (77 %) patients, with the infarcts distributed evenly between both hemispheres. The mean number of new lesions per patient was 4.2 ± 6.5 with a mean infarcted tissue volume of 2.05 ± 3.5 ml. The authors demonstrated that increasing age and aortic arch atheroma burden were independent predictors of new ischemic lesions post TAVR. General health status and quality of life improved at 30 days post TAVR with no functional cognitive decline recorded.
The significance of clinically silent cerebrovascular ischemic events post TAVR remains unclear. As detailed above DW MRI evaluation has shown that between 68 and 86 % of TAVR patients have evidence of acute ischemic insults [23–27]. General population studies have found that the prevalence of silent brain infarcts increases with age and may be as high as 28 %, fivefold more common than stroke [28–30]. Though the consequences of silent cerebral ischemia are not fully elucidated, they have been associated with visual defects, motor abnormalities, cognitive decline, and altered mood [31]. Further studies will have to elucidate the real clinical consequences of these DW MRI defects following TAVR procedures.
Pathophysiology of Cerebroembolic Events During TAVR (Box 20.1)
Box 20.1 Potential Etiologies of Cerebroembolic Events During TAVR
Aortic arch manipulation
Cerebral emboli (gaseous or thrombotic)
Showering of calcium during balloon aortic valvuloplasty
Balloon post-dilatation
Hypoperfusion
Atrial fibrillation
Aortic Arch Manipulation
Given the complexities of the TAVR procedure and the often coexistent multiple patient comorbidities, inadvertent cerebral embolization may result from a multitude of events. For instance, left heart catheterization alone via the TF approach, wherein a stenosed calcified aortic valve is crossed in its retrograde fashion, has been shown to result in frequent cerebroemboli [32]. Omran et al. [32] described the occurrence of clinically apparent stroke in 3 % and cerebral DW MRI changes consistent with acute ischemic lesions in 22 %, following retrograde AV crossing. Several studies employing cerebral DW MRI have shown asymptomatic ischemic lesions due to cardiac catheterization with an incidence ranging from 5 % to the 22 % of the aforementioned work [32–34]. Clinically overt stroke has been reported in 0.44 % of patients undergoing PCI and was associated with diabetes mellitus, hypertension, prior stroke, or renal failure [35]. However, age has been shown to influence this rate with one study showing a more than doubling of the stroke rate in patients older than 80 years (0.58 % vs. 0.23 %, p < 0.001) [36]. Perhaps of even more relevance to TAVR are the associations between aortic atheroma, aortic scraping during catheter manipulation, and release of microemboli [37–39]. Cerebral emboli may also be gaseous or originate from thrombi formed on the catheter or introducer sheath surface, in addition to displaced endovascular atheroma. The very large sheaths necessary for TAVR make smooth transitioning through the peripheral vasculature especially challenging. Furthermore, even with meticulous catheter preparation and flushing, air emboli are commonly observed using transesophageal echocardiography. A significant potential also exists with these catheters for air entrainment leading to large gaseous emboli [40].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree