Fig. 19.1
Tortuous iliofemoral arteries by angiography
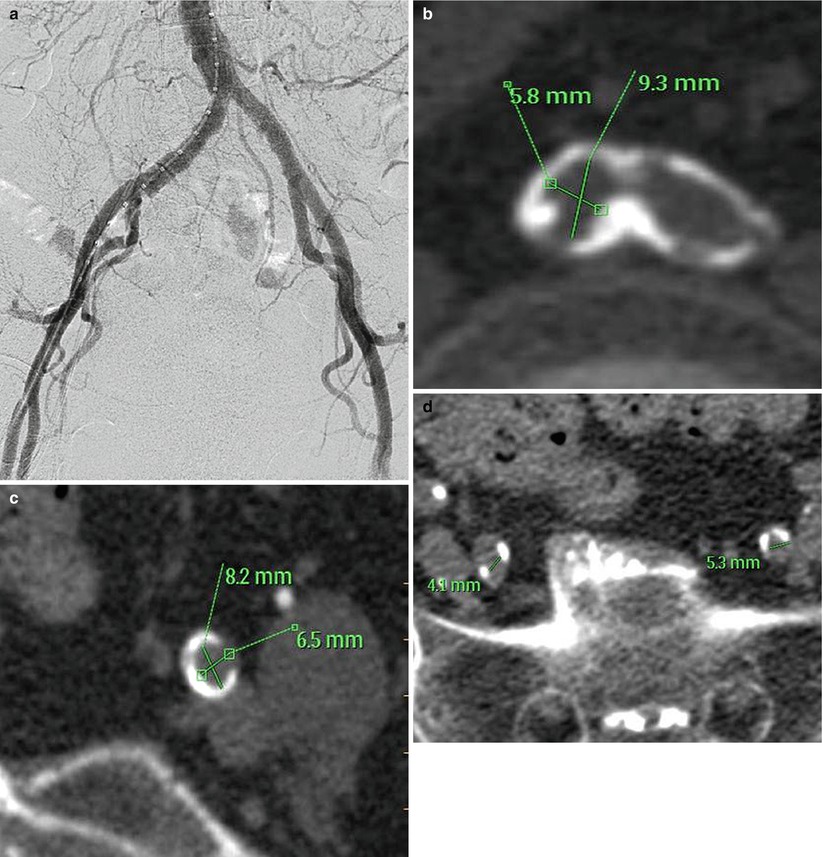
Fig. 19.2
(a) Aortoiliofemoral angiogram showing patent arteries with quantitative coronary angiography measurements >7 mm. (b) Bifurcation of right and left common iliac arteries showing severe circumferential calcification and small vessel size. (c) Circumferential calcification of left external iliac artery. (d) Heavy calcification and small lumen of bilateral external iliac arteries
Eltchaninoff et al. [11] suggested an “angiography alone” technique for assessing the suitability of iliac and femoral arteries for TAVR. They evaluated the iliac and femoral arteries of 135 patients by distal aortogram with bilateral iliofemoral runoff in the anteroposterior projection using a low dose of contrast (30 cc). Using a combination of vessel size, tortuosity, and calcification, these authors classified patients as suitable or not for the transfemoral approach. The authors proposed that patients who failed this “screening”—and thus were deemed unsuitable vessels according to the angiography—would not need to undergo CT angiography and, hence, receive additional contrast. These patients would instead be recommended to undergo TAVR via alternative routes.
In order to minimize contrast dose by angiographic evaluation, digital subtraction angiography is recommended. This method allows for same or better quality images with smaller amounts of contrast required (10–15 cc) (Fig. 19.3). Vessel diameters are measured with quantitative coronary angiography (QCA) and a reference marker pigtail. Precise vessel measurements are performed in multiple sites in the common femoral, superficial femoral, external, and common iliac arteries.
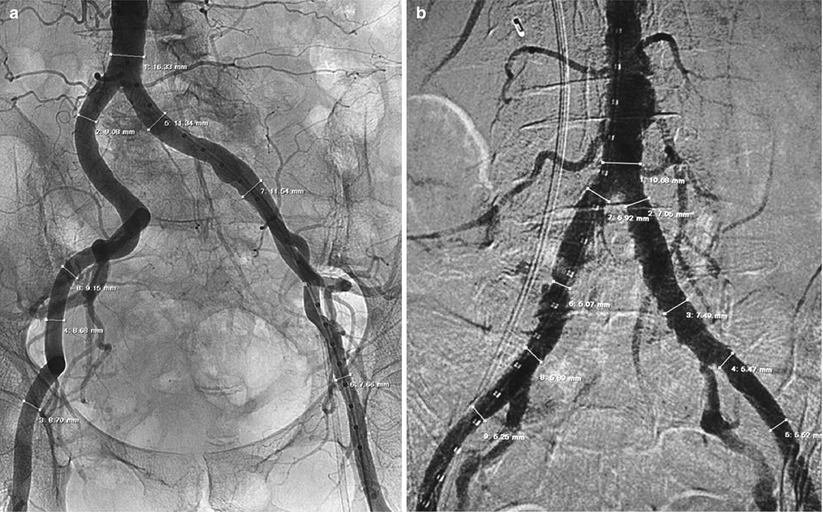

Fig. 19.3
(a) Abdominal aortography for measurement of vessel diameters with QCA and a reference marker pigtail. (b) Digital subtraction angiography for measurement of vessel diameters with QCA and a reference marker pigtail
Rotational digital subtraction angiography is a means to acquire multiple projection views during a single breath-hold injection. Seymour et al. [12] suggested that rotational digital subtraction angiography may provide reliable images of peripheral arteries. In their work, digital subtraction images were acquired every 3.5° in a rotational sweep of 120° to yield 33 projections to assess the renal arteries. The same principle can theoretically be applied to the iliofemoral arterial tree; however, limitations regarding measurement of lumen caliber and plaque morphology are likely to remain.
CT Angiography
Ultra-Low-Dose Intra-arterial Contrast Injection for Iliofemoral CT Angiography
A new method has been developed to perform CT angiography with significantly low contrast volume [13]. At the completion of the diagnostic cath, a 4-French pigtail catheter is positioned at the abdominal aorta, below the level of the renal arteries, and the patient is transferred to the CT suite. For the CT angiogram, iodinated contrast (iopamidol 370 mmol/ml) is mixed with normal saline in a 1:3 to 1:4 dilution in a single chamber of the power injector, and 40 ml of the contrast/saline mixture is injected through the pigtail catheter at 4 ml/s without a saline chaser. The scanning protocol incorporates a scan delay of 6 s followed by a helical CT of the abdomen and pelvis (64 × 0.625-mm collimation, rotation time 0.75 s, pitch 0.64, 120 kV, 154 mAs). With this technique it is possible to image the entire iliofemoral arterial tree with a very small amount of iodinated contrast (10–15 ml) (Fig. 19.4). Numerous technologic hardware innovations—including volume scanners with a greater number of detector rows, faster rotational speeds, iterative reconstruction method, and dual-energy CT imaging—may enable even lower doses of contrast to be required. Virtual endoscopy with CT is at times useful to estimate the distribution of calcium within the lumen along the vessel being considered for access (Fig. 19.5).
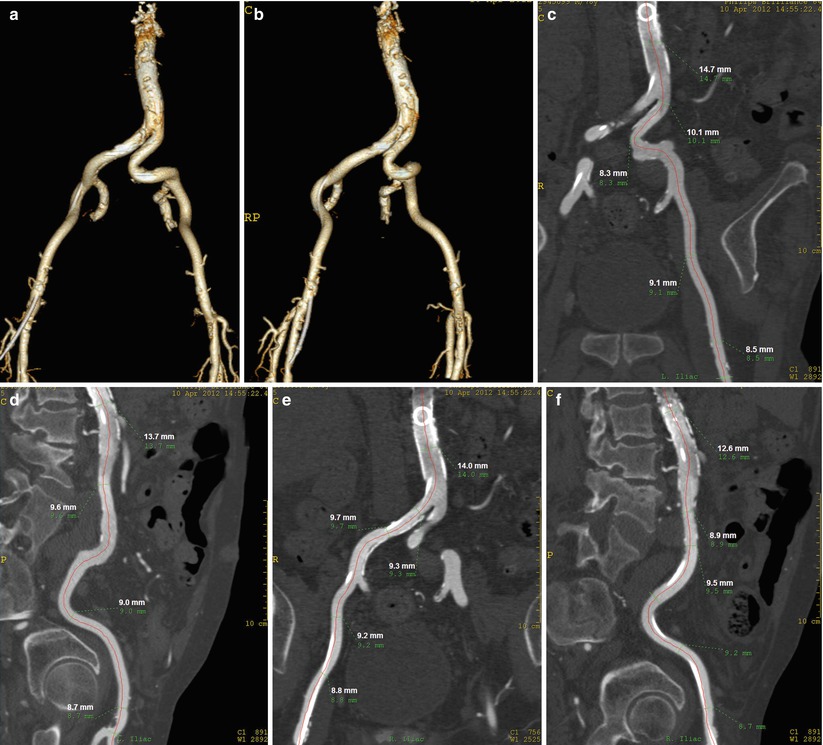
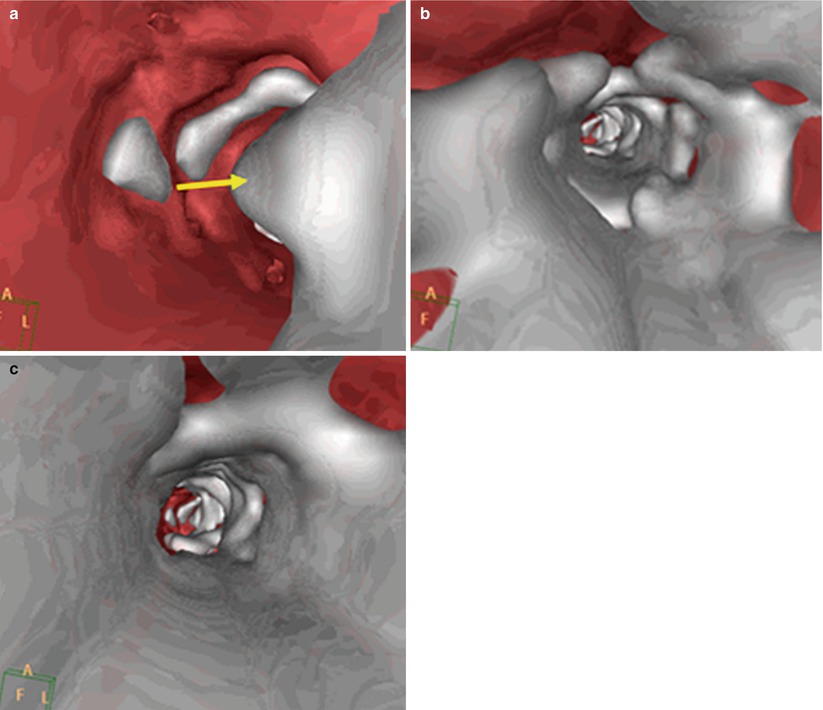

Fig. 19.4
(a) Images obtained with 10 cc of contrast injected in the abdominal aorta with a 4 F pigtail, AP view. (b) Right anterior oblique vie of the aortoiliofemoral arteries. This allows reconstructions in multiple views, which allows accurate measurements of the vessel diameters and the assessment of the calcification level. (c) Multiplanar reformation (MPR) of the left iliac artery in AP view. (d) MPR of the left iliac artery in lateral view. (e) MPR of the right iliac artery in AP view. (f) MPR of the right iliac artery in lateral view

Fig. 19.5
Virtual endoscopy with CT. (a) Vessel with small amount of calcium (arrow). (b) High amount of calcium with concentric arcs of calcium. (c) Severe circumferential calcification
Contrast-Saving Scanning Protocols
Technological advances in computed tomography imaging have improved spatial and temporal resolution without compromising on image quality (Box 19.1). These accomplishments were achieved through increases in the numbers of detectors and improved sampling capabilities in the z-direction. Improvements in image reconstruction, such as the development of iterative reconstruction techniques [14] and multi-segment reconstruction, have been shown to further enhance the signal-to-noise ratios and temporal resolution of cardiac computed tomography. Dual-source CT, which is constructed on the basis of two X-ray tubes and two detectors mounted on the CT gantry, with a mechanical offset of 90°, minimizes the limitations in temporal resolution of standard multidetector CT without the need for a faster gantry rotation. Using dual-source CT, Blanke et al. [15] were able to effectively study the aortic root anatomy and iliofemoral vasculature in a single acquisition using a single shot of 110–130 cc iodinated contrast and combined ECG-assisted scanning of the thorax and non-ECG-assisted scanning of the abdomen. Their experience indicates that adequate images could be achieved in nearly all patients using this method. Wuest et al. [16] further enhanced this protocol by using high-pitch spiral CT, which allows high-resolution imaging of large anatomical volumes in a short acquisition time. This technique also allows for the injection of significantly lower volumes of iodinated contrast (40 cc), which may be advantageous in patients with renal insufficiency or in high-risk AKI patients. Using high-pitch spiral CT, Wuest et al. were able to obtain all the standard aortic root and iliofemoral measurements required for the TAVR procedure.
Box 19.1 Reduced Iodinated Contrast CT Protocols
Direct intra-aortic injection | 4 Fr catheter inserted in infrarenal aorta |
Possible to perform iliofemoral assessment with 10–15 cc contrast | |
High-pitch helical CT | Short acquisition time significantly lowers contrast volume |
Dual energy CT | Monochromatic energy imaging may increase signal, thereby reducing contrast load |
Non-contrast CT
Non-contrast CT is very important for quantifying the amount of calcification in both longitudinal and axial views. In order to assess amount of calcification, the cursor is moved in a stepwise fashion (millimeter by millimeter) in search of significantly calcified areas. Significant calcification, especially in long arterial segments, does not allow for straightening. In addition, it is imperative to assess calcium distribution and localization at the puncture site since one of the most frequent modes of failure of percutaneous closure devices is when the device is deployed through a calcified vessel wall. Severe calcification at the bifurcation of internal and external iliac arteries is of special concern since this area has no yield for expansion or movement, that is, the internal iliac anchors the bifurcation.
“Blooming,” or partial volume, artifacts are frequent in non-contrast CT due to limited spatial resolution. Blooming artifacts require minimization in order to perform adequate vessel measurements and to quantify the calcification extent. The dimensions can change significantly after correction (Fig. 19.6).
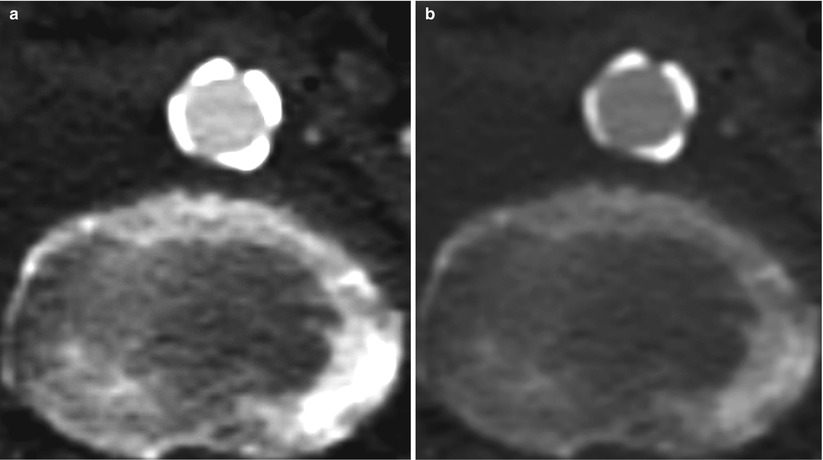

Fig. 19.6
High-level partial volume or “blooming” artifact that (a) distorts vessel diameter measurement and evaluation of calcium severity. Reducing the degree blooming (b) allows for more precise measurements
Several institutions assess the size of the iliofemoral arteries using non-contrast CT. Such an approach is not recommended, however, since the accuracy of measuring vessel size, especially in inexperienced hands, is limited and is a potential hazard for vascular complications during the procedure.
Intravascular Ultrasound
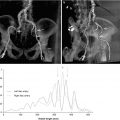
Intravascular ultrasound (IVUS) of the iliofemoral vessels can be used when there is a discrepancy in the angiography and CT results. IVUS is an excellent tool to measure vessel diameter and has been shown to significantly decrease the use of iodinated contrast (Fig. 19.7) [17]. However, this imaging modality has several limitations. IVUS does not allow for satisfactory calcification analysis and it may provide poor imaging in severely tortuous iliac arteries due to the position of the ultrasound probe within the artery lumen. In our institution, when high-quality CT images are available, IVUS images do not add appreciably useful additional information and, therefore, are used sparingly.
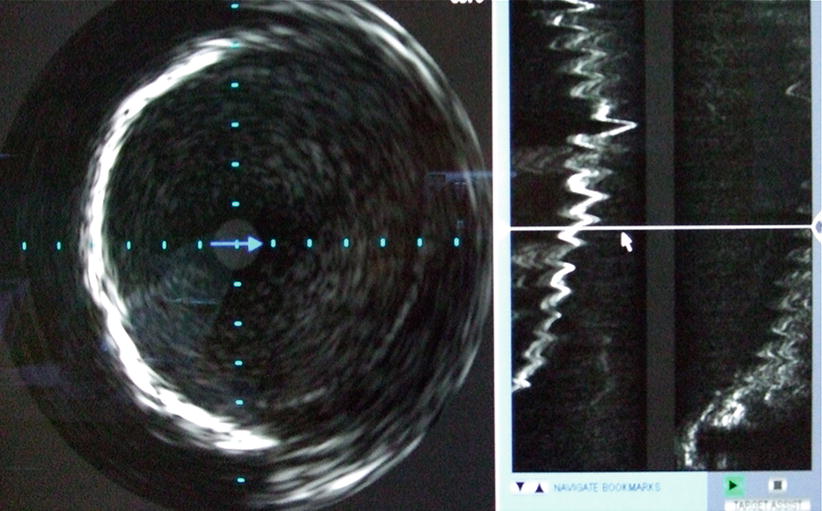

Fig. 19.7
Intravascular ultrasound to assess size and characteristics of iliofemoral vessels
Pre-procedural Evaluation: Aortic Valve Annulus Sizing
While gated CT angiography protocols are gaining acceptance for aortic valve annulus sizing as a pre-procedural planning tool [18], this modality requires the administration of large amounts of iodinated contrast and is thus not recommended for patients with renal insufficiency. An alternative screening tool which in our experience is sufficient in the majority of the cases is to assess the sub-annular diameter, left ventricular outflow tract in two-dimensional (2D) transthoracic echocardiography. In cases where this measurement is borderline or is technically difficult, transesophageal echocardiography is recommended. The perpendicular axis of the annulus using three-dimensional (3D) and/or biplane imaging is determined. Using these techniques, the diameter or perimeter of the aortic annulus is measured. It should be noted, however, that while 2D and 3D measurements are well correlated, 3D measurements, when compared to 2D measurements, typically yield more than 1-mm differences in mean dimensions [19].
Kempfert et al. [20] showed that end-systolic maximal annular diameter of the aortic valve was best correlated with direct intraoperative diameters measured after decalcification of the aortic annulus. These measurements also correlated well with CT “effective” diameter. As described above, several CT techniques are currently available to minimize contrast dose [15, 16].
Procedural Techniques
Generally, TAVR procedures do not require large amounts of iodinated contrast. The components of the procedure that typically require iodinated contrast injections are vascular access, aortic root angiogram to determine the perpendicular angulation for valve deployment, valve deployment for final positioning, post-deployment valve assessment, and the final iliofemoral assessment to exclude vascular access injury. There are various techniques that can be undertaken to perform any of these procedural stages without injecting contrast or with minimization of the amount of the injected contrast as outlined in Table 19.1.
Table 19.1




Contrast volume usage throughout the TAVR procedure
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree